Капельные пилюли для сердца «Фуфан Даньшэнь» (Fufang Danshen Diwan) 180 шт.
Эффективный, натуральный препарат для укрепления сердца, предотвращения инфаркта миокарда, от стенокардии и других заболеваний сердца.
Применяется для лечения стагнации Ци и крови, а также возникших на фоне этого недостаточности дыхания и боли в грудной полости и сердце, чувства стеснения в груди
Действие препарата:
- расширяет коронарные артерии,
- усиливает сократительную способность миокарда,
- улучшает питание сердечной мышцы,
- тормозит коагуляцию тромбоцитов,
- усиливает кровоток,
- улучшает микроциркуляцию,
- оказывает также противоаритмическое действие.
Показания к применению:
- ишемическая болезнь сердца,
- острый инфаркт миокарда,
- стенокардия (нестабильная, коронароспастическая; стабильная, ночная),
- кардиомиопатия (гипертрофическая, обструктивная),
- кардиосклероз,
- миокардиодистрофия,
- брадикардия,
- тахикардия,
- перикардит,
- ревмокардит,
- гипертоническая болезнь,
- сердечная недостаточность,
- заболевания артерий и вен,
- синдром Рейно,
- спазмы периферических артерий.
Состав: корень и корневище шалфея, женьшень ложный, борнеол, Panax notoginseng, и т.д.
Способ применения: по 10 пилюль 3 раза в день за 30 мин до еды. Рассасывать под языком более эффективно.
Рекомендуемый курс: 28 дней,
4-5 упаковок.
Противопоказания: беременность, лактация, аллергия на компоненты.
Упаковка: 180 пилюль х 27 мг.(бутылочка с дозатором)
Побочные действия: в редких случаях, дискомфорт в желудке и кишечнике.
Хранение: в тёмном прохладном месте.
Срок годности: 48 месяцев.
Не является лекарством.
Номер разрешения: Z10950111
Производитель: TASLY PHARMACEUTICAL GROUP CO .. LTD, производственный адрес: No. 2 Puji River East Road, район Бэйчэн, Тяньцзинь, Китай.
Вариант 1: Почта России( точная сумма за доставку определяется после сбора заказа)
Внимание! Стекло, хрупкое. Если клиент не заказал обрещётку груза, ответственность за сохранность груза лежит на нём. Мы не можем отвечать за качество и бережное отношение к грузу сторонних организаций осуществляющих доставку.
Отправка Заказа от 1-3 рабочих дней !
Отправка Заказа может задержаться по причине отсутствия товара на складе
( ожидание !!!! ) Просим Нас понять, так как препараты идут в Москву с Индии , Китая, Тайланда .
( мы их не делаем в Москве) и проходят длительный путь !
При наложенном платеже вы оплачиваете товар в момент получения его на почте.
При отправке посылки по предоплате, вы экономите:
1. на оплате тарифа за перевод наложенного платежа
2. на тарифе за объявленную ценность.
Ориентировочный расчет стоимости почтовых отправлений в регионы РФ — на сайте Почты России
Для Дальних Регионов, как Магадан и область, Якутия, Норильск, Камчатка,СНГ и другие — расчет стоимости индивидуальный через Ориентировочный расчет стоимости почтовых отправлений в регионы РФ — на сайте Почты России
Сумма за доставку в накладной — Программой выдаётся приблизительная , корректируется оператором После взвешивания заказа и оформления Документов на груз!
Стоимость доставки: 0 рублей
Вариант 2: СДЕК. До Вашего Города- за счет Получателя.
До Вашего Города — за счет Получателя.
Страны СНГ оплачивают Доставку Предварительно!
Стоимость доставки: 0 рублей
Вариант 3: Курьер по Москве
Доставка курьером производится в пределах МКАД.
Если Ваш Заказ:
более 5 кг — Доставка 500 руб
более 10 кг — Доставка 750 руб
Оплата : Наличные или ОнЛайн перевод на карту. Терминал есть только в Магазине!
Стоимость доставки: 350 рублей
Вариант 4: Самовывоз с САЛОН-МАГАЗИН «Секреты Лан»
СДЕЛАТЬ ЗАКАЗ ПО ТЕЛЕФОНУ
Вы можете заказать товары по телефону с помощью оператора интернет-магазина.
Заказы по телефону 8 925 199 32 87 принимаются с 10 до 20 часов с понедельника по пятницу включительно.
г. Москва, м. Каховская, Симферопольский бульвар, д. 15, корпус 1
Стоимость доставки: 0 рублей
Отзывов пока не было. Вы можете оставить его первым
1 Introduction
Globally, coronary heart disease (CHD) primarily causes cardiovascular-related deaths, with nearly 7.3 million annual fatalities reported, out of which approximately 130,000 have been reported in China.[1–3] Adopting preventive measures that emphasize healthy habits (physical activity, diet, and not smoking) and following secondary prevention medications reduces the incidence of CHD mortality by at least 47% and reduce 68% of CHD-related primary risk factors.[4–6] The main characteristics of CHD include coronary atherosclerosis lesions caused by myocardial ischemia, necrosis or hypoxia, occlusion of the lumen, stenosis, and acute inflammation.[7] Recently, 2 types of syndromes were clinically proposed to keep continuously updating the diagnosis and therapy for CHD and formulate therapeutic approaches, including, acute coronary syndrome and chronic myocardial ischemia syndrome.[8,9] Improving myocardial blood supply is vital to clinically treat CHD, which usually involves the intake of antiplatelet agents. Despite advances in modern clinical practice, CHD still has a high prevalence, particularly in developing nations, the disease strains the medical sector, and incurs a substantial financial burden on society, impeding human development.[10,11]
In accordance with the fundamental theories of conventional Chinese medicine, CHD’s etiology and pathogenesis are linked to blood stasis. Thus, removing blood stasis by promoting blood circulation are primary methods to treat CHD. The compound Danshen dropping pill (CDDP) is primarily composed of Salvia miltiorrhiza Bunge, Panax notoginseng, and Borneol. It is effective to improve circulation, removal of stasis, vital energy regulation, and alleviating pain, with particular significance for clinically treating cardiovascular diseases.[12] Improving blood circulation to regulate qi-flowing and eradicate blood stasis to alleviate physical pains are the primary functions of CDDP.[12] It has been reported that CDDP has a considerably healing effect on vascular endothelial functions, relieving angina pectoris and averting restenosis following coronary stenting. However, there is a lack of studies on its effect on coronary artery lesions. Therefore, the present systematic analysis is conducted to evaluate the efficacy and safeness of using CDDP as a treatment for CHD cases.
2 Objectives
This research protocol aims to outline a methodical analysis and methodical evaluation to examine the effectiveness and safeness of administering CDDP to treat CHD patients.
3 Methods and analysis
The present protocol will be conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) statement.[13] This protocol has been registered in the Open Science Framework (https://osf.io/), registration number: 10.17605/OSF.IO/HJTP8.
4 Criteria for considering studies for this review
4.1 Types of studies
The proposed systematic review shall consider all randomized controlled trials (RCTs) that examine the effectiveness and safeness of administering CDDP to treat CHD patients.
4.2 Types of participants
All participants having CHD will be included, the diagnosis of CHD should adhere to the CHD diagnosis criteria of the clinical diagnosis standards specified by the World Health Organization (WHO), regardless of nationality and gender.[14]
4.3 Types of interventions
The experimental group will be treated with CDDP, irrespective of the dose, meanwhile, the controls shall be administered other treatment, except CDDP treatment; however, the intake of non-lipid-lowing drugs and non-anticoagulants will be permitted.
4.4 Types of outcome measures
Outcome measures include severe adversities related to hemorheology indices, cardiac events, vascular endothelial function indicators, blood lipid parameters, cardiac function indicators, cardiac index, and adverse reactions.
5 Search methods for identification of studies
RCTs that assess the effectiveness and safeness of using CDDP to treat CHD patients will be searched in MEDLINE, Cochrane Library, China National Knowledge Infrastructure, EMBASE, and WanFang databases inception to January 3, 2022. The combination of the following search terms will be used: (“compound Danshen dripping pill∗” OR “Fufang Danshen diwan” OR CDDP) AND (“coronary heart disease” OR “CHD” OR “coronary artery disease” OR “CAD”) AND (“randomized controlled trials” OR “randomized controlled studies” OR RCT). Another search will be performed to obtain conference proceedings and the reference lists included in review articles to obtain additional articles. There will be no constraints on language or publication period.
6 Data collection and analysis
6.1 Selection of studies
The described search strategy shall be employed to source titles and abstracts that could be related to the present review. Subsequently, 2 authors will separately screen the titles and abstracts, and illegible articles will be discarded. All eligible studies and reviews will be retained at this stage.
6.2 Data extraction and management
A pair of reviewers will separately evaluate all the obtained abstracts and, if needed, the complete text to ascertain which studies meet the criteria for inclusion. The same authors will perform data extraction independently using standardized data extraction forms. All non-English studies will be translated prior to assessment. In instances where there is more than one publication for a study, we will group the reports and the most updated data set (recent) was considered for analysis. We will highlight all disparities between published versions.
6.3 Assessment of risk of bias in included studies
We will assign 2 independent authors to review the systematic quality of the eligible trials using the Cochrane Collaboration’ s tool for bias risk assessment.[15] The tool evaluates the presence of bias in selection by considering the randomization procedures and distribution suppression, performance, and detection of bias by examining the blinding of personnel and outcome assessment, and attrition and bias in reporting by assessing partial and selective data reporting. Each item will be allocated a judgement of high, low, or unclear risk.
6.4 Measures of treatment effect
We will express the results as the relative risk with 95% confidence intervals for dichotomous outcomes. Meanwhile, the mean difference will be used to assess treatment effects for continuous scales of measurement. However, if different scales are used, the results will be presented as standardized mean difference (SMD).
6.5 Assessment of heterogeneity
A chi-squared test will be adopted on N-1 degrees of freedom to analyze the heterogeneity (α = 0.05 denotes statistical significance) and the I2 test.[15]I2 values of 25%, 50%, and 75% indicate low, medium, and high levels of heterogeneity, respectively.
6.6 Assessment of reporting biases
In the case that a large enough number of trials were recognized, funnel plots will be adopted to examine bias in reporting.[16]
6.7 Sensitivity analysis
We will perform a sensitivity analysis to determine the robustness of our results. Accordingly, we shall omit articles associated with an elevated bias risk from the summary analysis and evaluate the studies by repeating the evaluation to assess how the studies impact the results.
7 Discussion
Globally, CHD is a predominant reason behind morbidity and mortality.[4,17,18] In Western clinical practices, the administration of lifelong aspirin and clopidogrel therapy are regarded as effective ways to reduce fatalities from cardiac problems, myocardial infarction, and strokes.[19] However, there are limitations in dual Western medical antiplatelet therapy, as the abrupt premature stoppage elevates myocardial infarction risk and fatal outcomes.[2,9] Therefore, we will search all related literature without any language constraints to include all relevant trials of CDDP for CHD. The present systematic review and meta-analysis will summarize existing evidence on the effectiveness and safeness of using CDDP to treat CHD patients. The evidence will be a useful reference for clinical practitioners, patients, and health policymakers.
Author contributions
Conceptualization: Ling Sun, Yanna Zhang.
Data curation: Ling Sun, Yanna Zhang.
Formal analysis: Ling Sun, Yanna Zhang.
Funding acquisition: Ling Sun, Yanna Zhang.
Methodology: Yanna Zhang.
Project administration: Ling Sun.
Resources: Ling Sun, Yanna Zhang.
Software: Ling Sun.
Supervision: Yanna Zhang.
Validation: Ling Sun, Yanna Zhang.
Visualization: Yanna Zhang.
Writing – original draft: Ling Sun, Yanna Zhang.
Writing – review & editing: Yanna Zhang.
References
[1]. Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol 2010;35:72–115.
[2]. Bechthold A, Boeing H, Schwedhelm C, et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr 2019;59:1071–90.
[3]. World Health Organization, Mendis S, Puska P, Norrving B, Organization WH. Global Atlas on Cardiovascular Disease Prevention and Control. 2011.
- Cited Here
[4]. Devaraj SM, Kriska AM, Orchard TJ, Miller RG, Costacou T. Cardiovascular health in early adulthood predicts the development of coronary heart disease in individuals with type 1 diabetes: 25 year follow-up from the Pittsburgh Epidemiology of Diabetes Complications study. Diabetologia 2021;64:571–80.
[5]. Lv J, Yu C, Guo Y, et al. Adherence to healthy lifestyle and cardiovascular diseases in the Chinese population. J Am Coll Cardiol 2017;69:1116–25.
[6]. Han C, Liu F, Yang X, et al. Ideal cardiovascular health and incidence of atherosclerotic cardiovascular disease among Chinese adults: the China-PAR project. Sci China Life Sci 2018;61:504–14.
[7]. Pencina MJ, Navar AM, Wojdyla D, et al. Quantifying importance of major risk factors for coronary heart disease. Circulation 2019;139:1603–11.
[8]. Kachur S, Chongthammakun V, Lavie CJ, et al. Impact of cardiac rehabilitation and exercise training programs in coronary heart disease. Prog Cardiovasc Dis 2017;60:103–14.
[9]. Świątkiewicz I, Di Somma S, De Fazio L, Mazzilli V, Taub PR. Effectiveness of intensive cardiac rehabilitation in high-risk patients with cardiovascular disease in real-world practice. Nutrients 2021;13:
[10]. Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016;4:256.
[11]. Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001;104:2746–53.
[12]. XD ME, Cao YF, Che YY, et al. Danshen: a phytochemical and pharmacological overview. Chin J Nat Med 2019;17:59–80.
[13]. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:01.
[14]. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020;41:407–77.
[15]. The Cochrane Collaboration, Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. 2013.
- Cited Here
[16]. John Wiley & Sons, Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2019.
- Cited Here
[17]. Reddy KS. Cardiovascular disease in non-Western countries. N Engl J Med 2004;350:2438–40.
[18]. Yeates K, Lohfeld L, Sleeth J, Morales F, Rajkotia Y, Ogedegbe O. A global perspective on cardiovascular disease in vulnerable populations. Can J Cardiol 2015;31:1081–93.
[19]. Kinlay S, Quach L, Cormack J, et al. Premature discontinuation of dual antiplatelet therapy after coronary stenting in veterans: characteristics and long-term outcomes. J Am Heart Assoc 2021;10:e018481.
Keywords:
compound Danshen dripping pills; coronary heart disease; efficacy; meta
- Research
- Open access
- Published:
- Hong-Min You2 na1,
- Chang-Zhen Ren1 na1,
- Bo-Wen Hu1,
- Lu-Jun Zhang2,
- Yan-Da Zhang1,
- Zhi-Qing He1,
- Ru Ding1,
- Zhi-Fu Guo2 &
- …
- Chun Liang1
BMC Complementary Medicine and Therapies
volume 22, Article number: 112 (2022)
Cite this article
-
2910 Accesses
-
2 Altmetric
-
Metrics details
Abstract
Background
The compound Danshen Dripping Pill (CDDP), which is a mixture of extracts from Radix Salviae and Panax notoginseng, is a patented traditional Chinese medicine that is widely used in multiple countries for relieving coronary heart disease (CHD), but its pharmacological mechanism has not been fully elucidated. In this study, we screened the key pharmacological pathways and targets of CDDP that act on CHD using a network pharmacology-based strategy, and the angiogenic activity of CDDP was directly visually investigated in zebrafish embryos in vivo.
Methods
The potential therapeutic targets and pathways were predicted through a bioinformatics analysis. The proangiogenic effects of CDDP were examined using vascular sprouting assays on subintestinal vessels (SIVs) and optic arteries (OAs) as well as injury assays on intersegmental vessels (ISVs). Pharmacological experiments were applied to confirm the pathway involved.
Results
Sixty-five potential therapeutic targets of CDDP on CHD were identified and enriched in the PI3K/AKT and VEGF/VEGFR pathways. An in vivo study revealed that CDDP promoted angiogenesis in SIVs and OAs in a dose-dependent manner and relieved the impairments in ISVs induced by lenvatinib, a VEGF receptor kinase inhibitor (VRI). In addition, Vegfaa and Kdrl expression were significantly upregulated after CDDP treatment. Furthermore, the proangiogenic effect of CDDP could be abolished by PI3K/AKT pathway inhibitors.
Conclusions
CDDP has a proangiogenic effect, the mechanism of which involves the VEGF/VEGFR and PI3K/AKT signaling pathways. These results suggest a new insight into the cardiovascular protective effect of CDDP.
Peer Review reports
Introduction
Current data from the World Health Organization show that cardiovascular diseases (CVDs) are still the leading cause of death among noncommunicable diseases and account for approximately one-third of all deaths worldwide [1]. Among CVDs, coronary heart disease (CHD) may cause angina pectoris (AP), which signals the risk of myocardial infarction and sudden death and is also recognized as a predictor of many other major adverse cardiac events. However, therapies for CHD and other ischemic CVDs are limited.
Angiogenesis is a process by which new blood vessels sprout from pre-existing blood vessels and is affected by VEGF/VEGFR signaling [2]. Endothelial cells (ECs), with physiological functions such as proliferation, migration, and tube formation play a key role in the initiation of angiogenesis [3]. Drugs targeting both proangiogenic and antiangiogenic processes have been developed to help maintain the normal physiological condition of ECs [4]. In recent decades, to suppress the nutrient supply of tumors, antiangiogenic drugs have been widely applied in cancer treatment [2], and proangiogenic therapy is important for treating and preventing the serious consequences of CHD [5]. Enhancing angiogenesis facilitates the delivery of oxygen and nutrients to the ischemic site of cardiomyocytes, which benefits the process of myocardial healing.
Compound Danshen Dripping Pill (CDDP), a patented traditional Chinese medicine (TCM), is widely used for relieving AP, ischemic heart diseases and coronary arteriosclerosis in China. It was first launched in 1995 by the China Food and Drug Administration (CFDA) and was approved by the US Food and Drug Administration (FDA) in 2016 to enter phase III (NCT01659580) clinical trials [6]. Currently, CDDP is marketed as a prescription or nonprescription drug for the prevention and treatment of ischemic CVDs in Singapore, Canada, South Korea, Mongolia, and Vietnam. However, the mechanism of CDDP in the treatment of CHD has not been fully elucidated. According to previous reports, CDDP is a mixture of extracts from Radix Salviae (Danshen), Panax notoginseng (Sanqi), and other herbs [7]. These active components of CDDP have been reported to improve microcirculation in ischemic/reperfusion injury and other CVDs [8], and we recently discovered that pretreatment with CDDP prevents lipid infusion-induced cardiac microvascular disorder (CMD) in a mouse model [9]. However, whether angiogenesis is involved in the cardiovascular protective effect of CDDP remains unknown.
The zebrafish has become an ideal animal model to study angiogenesis, in part because its responses to many pro- or antiangiogenic drugs are comparable to those observed in mammalian systems [10,11,12]. Remarkably, the formation of the cardiovascular system is highly typical in developing zebrafish larvae, and the subintestinal vessel plexus (SIVs), intersegmental vessels (ISVs) and optic arteries (OAs) can be visualized microscopically [13, 14]. Furthermore, the zebrafish embryo assay allows the identification of side effects, including toxicity and developmental delay [15]. In this study, we screened the key pharmacological pathways and targets of CDDP that act on CHD using a network pharmacology-based strategy. The angiogenic activity of CDDP was then directly visually investigated in zebrafish embryos in vivo. Our results provide novel evidence that CDDP exhibits proangiogenic activity by inducing the formation of new blood vessels and stimulating endothelial cell proliferation; hence, CDDP could also serve as a remedy targeting VEGF/VEGFR and PI3K/AKT signaling for ischemic CVDs.
Materials and methods
Network pharmacology-based screening
The main active ingredients of CDDP, Radix Salviae and Panax notoginseng, were recognized based on traditional medicine guidelines and the Chinese Clinical Trial Registry up to April 30, 2020 (all components are listed in Supplemental Table 1). The constituents were screened using the Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) (https://old.tcmsp-e.com/tcmsp.php) with the filters OB (oral bioavailability) ≥ 30% and DL (drug-likeness) ≥ 0.18. Target prediction of the active compounds was then performed using TCMSP and confirmed through the DrugBank database (https://go.drugbank.com/). For CHD-related target screening, we searched the keyword ‘coronary heart disease’ in the GeneCards database (https://www.genecards.org/). The target genes obtained were then pooled using R software, and the genes in common were regarded as hub genes to be further screened. The protein–protein interactions of these hub genes were constructed using the STRING database (http://string-db.org/) with a minimum required interaction score of 0.950. The Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) [16] pathway enrichment analyses were performed and visualized using R software.
Chemicals and reagents
CDDP extracts were purchased from Tasly Pharmaceutical Group Co. Ltd. (Tianjin, China, 27 mg/package, batch number 20210103) and dissolved using dimethyl sulfoxide (DMSO) (Sigma–Aldrich, Munich, Germany) to make different concentrations. In previous research, the chemical profile of CDDP had been fully investigated using HPLC [17]. The final DMSO concentration was ≤ 0.5% weight/volume (w/v) in all treatment groups. The VEGF receptor kinase inhibitor (VRI) lenvatinib, PI3K inhibitor LY294002 (LY) and AKT inhibitor VIII (AKTi) were obtained from Beyotime Technology (Shanghai, China).
Zebrafish maintenance and feeding
Transgenic zebrafish embryos, Tg(fli1: EGFP), were obtained from the Laboratory of the Department of Cardiology, Shanghai Changhai Hospital, maintained at 28 °C on a 14/10-h light/dark cycle and staged as previously described [18]. To prevent pigmentation during embryonic development, 0.003% 2-phenylthiourea (PTU, Sigma–Aldrich, CA, USA) was added to the culture water. The development age of the embryos corresponded to hours post-fertilization (hpf) and was staged according to Kimmel et al. [19]. The embryos applied in the experiments were all normally developed and reached the indicated stages. Approximately one hundred embryos were collected in a 6 cm dish and treated with Danieu’s solution with 0.04% tricaine (Sigma–Aldrich, CA, USA) for immobilization before the corresponding assays.
Vascular sprouting assay
Twenty-four hpf WT zebrafish were used to perform toxicity screening of CDDP. After being dechorionated with 0.5 mg/mL Pronase E (Roche, IN, USA), 24 hpf Tg(fli1:EGFP) embryos were incubated with CDDP at safe concentrations (0.3, 0.6, 0.9 and 1.2 mg/mL) for 48 h. DMSO (≤ 0.5%) served as the vehicle control and was equivalent to no treatment. For each concentration, three replicates of fifteen embryos were set up and exposed to 1 mL of test medium. The entire screening system was performed in a 24-well tissue culture plate. At 72 hpf, the embryos were examined using a fluorescence microscope (Leica, Wetzlar, Germany) and each group tested was scored for mortality and morphological defects. The numbers of sprouting vessels in SIVs and abnormally developed vessels in OAs were photographed and counted for quantitative analysis. For the ISV injury and restoration assay, twenty-four hpf zebrafish embryos were treated with VRI (100 nM) for 24 h and then incubated with or without various concentrations of CDDP for another 24 h. LY and AKTi were added to the system 30 min before CDDP when used. The intact and deficient vessels of intersegmental vessels (ISVs) were calculated for each embryo. The ISV index was calculated as: (the number of intact vessels × 1) + (the number of defective vessels × 0.5).
Total RNA extraction and real-time quantitative PCR (rt-qPCR)
Total RNA was isolated from CDDP-treated embryos using TRIzol reagent (Thermo Fisher, MA, USA) and reverse transcribed using a FastQuant RT Kit (Tiangen, Beijing, China). Quantitative RT–PCR assays were performed using SYBR Green Real-time PCR Master Mix (Toyobo, Osaka, Japan). The primers used for mRNA detection are listed in Supplementary Table 2 and were provided by Biosune Ltd., Shanghai, China.
Statistical analysis
All experiments were conducted more than three times. The data are presented as the mean ± SEM and were analyzed using GraphPad Prism 7.0 software. Normality was evaluated using the Shapiro–Wilk test. The Mann–Whitney test or Kruskal–Wallis test followed by Dunn’s multiple comparisons test was then performed for the data that did not fit a normal distribution. Whereas for the data that followed a normal distribution, Levene’s test of homogeneity of variance was further performed. When the data exhibited homogeneity of variance, a one-way ANOVA was applied. A P < 0.05 was considered statistically significant.
Results
Screening of potential target genes and pathways of CDDP
The target genes of the two ingredients of CDDP, Radix Salviae and Panax notoginseng, were screened using the TCMSP database. A total of 65 compounds were identified in Radix Salviae (Supplementary Table 3), and 8 were identified in Panax notoginseng (Supplementary Table 4). We then obtained known targets of these ingredients, which are listed in Supplementary Table 5 and Supplementary Table 6, from the TCMSP databases. Furthermore, 7,100 CHD-related genes were obtained from GeneCards databases (Supplementary Table 7). A Venn plot revealed that 65 CHD-related genes were also targeted by CDDP (Fig. 1A, Supplementary Table 8). The count of the ingredients predicted to regulate each of the target genes showed that AKT1 and JUN involved the most constituents of CDDP (Fig. 1B). The protein–protein interaction (PPI) network of these 65 target genes was constructed, and nodes with high confidence were identified by limiting their interaction scores to more than 0.950 and excluding the separated proteins (Fig. 1C). We noticed that VEGFA and AKT1 were both predicted to be hub proteins in the PPI network. GO analysis of the overlapping targets showed that DNA-binding transcription factor binding, RNA polymerase II-specific DNA-binding transcription factor binding, and ubiquitin-like protein ligase binding were the top three enriched functions (Fig. 1D). In addition, 13 proteins were associated with DNA-binding transcription factor binding activity with a gene ratio of 0.231 (Fig. 1E). Thus, CDDP compounds might potentially target these proteins to regulate kinase activity, in particular the DNA-binding transcription factor binding process. As shown by the KEGG analysis, the top three significantly enriched pathways among the 65 overlapping genes were PI3K/AKT (18 proteins), Kaposi sarcoma-associated herpesvirus infection related (16 proteins), and Hepatitis B related signaling pathways (15 proteins), (Fig. 1F). The PI3K/AKT pathway ranked first with 18 proteins (Fig. 1G) and has been reported to play an important role in angiogenesis [20]. Moreover, VEGFA, one of the most powerful activators of VEGF/VEGFR signaling, was also identified as a hub protein in the PPI network above (Fig. 1C). We surmised that both the VEGF/VEGFR and PI3K/AKT pathways were targeted by CDDP in treating CHD.
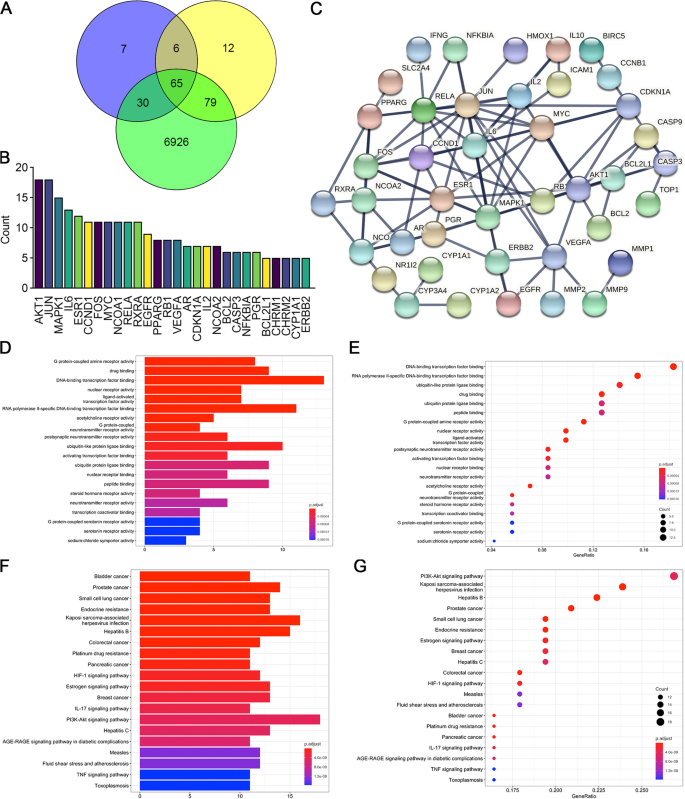
Screening of potential target genes andpathways of CDDP. A We obtained 7,100 coronary heart disease-related genes from GeneCards, 108 target genes of Panax notoginseng and 162 target genes of Radix Salviae from the TCMSP database. The 65 intersecting genes were considered important targets and were further studied. B Among the top 28 potential target genes predicted to be regulated by CDDP, AKT1 and JUN ranked highest. C Protein–protein interactions (PPIs) of the 65 targets (interaction score ≥ 0.950). The separated proteins were predicted to be less related and then deleted. D, E GO enrichment analysis showing the top 20 molecular biological functions of the target genes. This result demonstrated that DNA-binding transcription factor binding was the most common gene function. The abscissa represents the ratio of related genes, and the ordinate lists the functions corresponding to proteins. The bubble size represents the number of genes, whereas the color represents the P value. F, G KEGG pathway enrichment analysis showing the 20 most likely pathways involved; the PI3K-AKT signaling pathway ranked first
Full size image
The proangiogenic effect of CDDP on SIVs
We employed the transgenic zebrafish line Tg(fli1: EGFP), which specifically expresses enhanced green fluorescence protein (EGFP) in vascular endothelial cells, to evaluate the proangiogenic effect of CDDP. The toxicity of CDDP in zebrafish embryos was evaluated as follows. Twenty-four hours post-fertilization (24 hpf), embryos were treated with various concentrations of CDDP for 48 h because angiogenic vessel development begins at approximately 20 hpf and is completed at 72 hpf [21]. Figure 2A demonstrates that the survival rate decreased with CDDP concentration. The concentration of CDDP causing death to 50% of the tested embryos, or the median lethal concentration (LC50), was calculated to be 5.081 mg/mL. Figure 2B shows that up to 1.2 mg/mL, no significant morphological change in larvae exposed to the tested concentrations of CDDP could be found. Their body length (Fig. 2C) and yolk sac area (Fig. 2D), the indicators reflecting development and nutritional status, respectively, did not change significantly compared with those of the control group. Moreover, the observation of heart rate also showed insignificant differences (Fig. 2E). These results revealed that CDDP did not affect embryonic development during CDDP treatment.
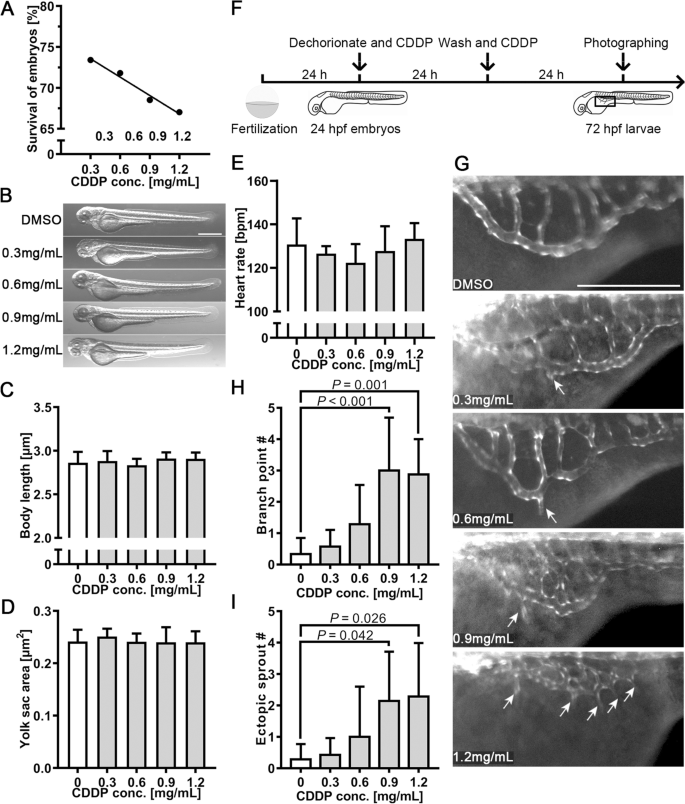
The proangiogenic effect of CDDP on subintestinal vessels (SIVs). A Twenty-four hpf embryos were treated with various concentrations of CDDP (0.3, 0.6, 0.9 and 1.2 mg/mL) for 48 h. The survival rate was recorded. B The toxicity screening of CDDP did not significantly affect the early-stage appearance of embryos. Scale bar, 50 μm. C Body length was not affected by CDDP treatment, indicating that embryo development was normal. D The yolk sac area was also unchanged, which meant that the nutritional status was maintained at a similar level. E Heart rates of the treatment groups were not notably different from those of the control group. F Schematic representation of the experimental design for G-, I. Twenty-four hpf transgenic zebrafish Tg(fli1: EGFP) embryos were cotreated with CDDP (0.3, 0.6, 0.9 and 1.2 mg/mL) for 24 h. After washing with culture water, the embryos were treated with the same concentration of CDDP for another 24 h. The box in 72 hpf larvae indicates the position photographed and displayed in G. G Representative pictures of each group; arrows indicate branch points and sprouting vessels of SIVs induced by CDDP in zebrafish. Scale bar, 50 μm. H, I The branch point and sprouting vessel numbers of SIVs were recorded in each zebrafish. Fifteen embryos were included in each group, and the experiment was replicated four times. Data are represented as the mean ± SEM
Full size image
Twenty-four hpf embryos were treated with CDDP as illustrated in Fig. 2F. As the results indicated in Fig. 2G, CDDP increased the branch point number and promoted ectopic vascular sprouting from SIVs (arrows). The count of SIV branch points (Fig. 2H) and ectopic sprouts (Fig. 2I) showed a significant difference between the control group and the CDDP treatment group at 0.9 and 1.2 mg/mL. These results revealed that CDDP enhanced the growth of SIVs in zebrafish.
CDDP induced endothelial layer thickening and ectopic growth of OAs
We also observed thickening of the walls of OAs (Fig. 3A-E) and noticed more ectopic growth of OAs in the 1.2 mg/mL CDDP treatment group (indicated by arrows in Fig. 3F-G). The thickness of the endothelial layer of OAs (T) was determined by subtracting the inner diameter (Di) from the outer diameter (Do) using the formula T = (Do—Di)/2 (Fig. 3H). Quantitative analysis confirmed the thickening of OAs in the group treated with 1.2 mg/mL CDDP (Fig. 3I). We further detected changes in the mRNA expression of Vegfaa (an isoform of VEGFA in zebrafish) and its receptors, Flt1, Kdrl and Flt4, in the whole tissue homogenate of CDDP-treated embryos. Figure 3J demonstrates that the expression levels of both Vegfaa and Kdrl mRNA were upregulated in the 1.2 mg/mL CDDP treatment group.
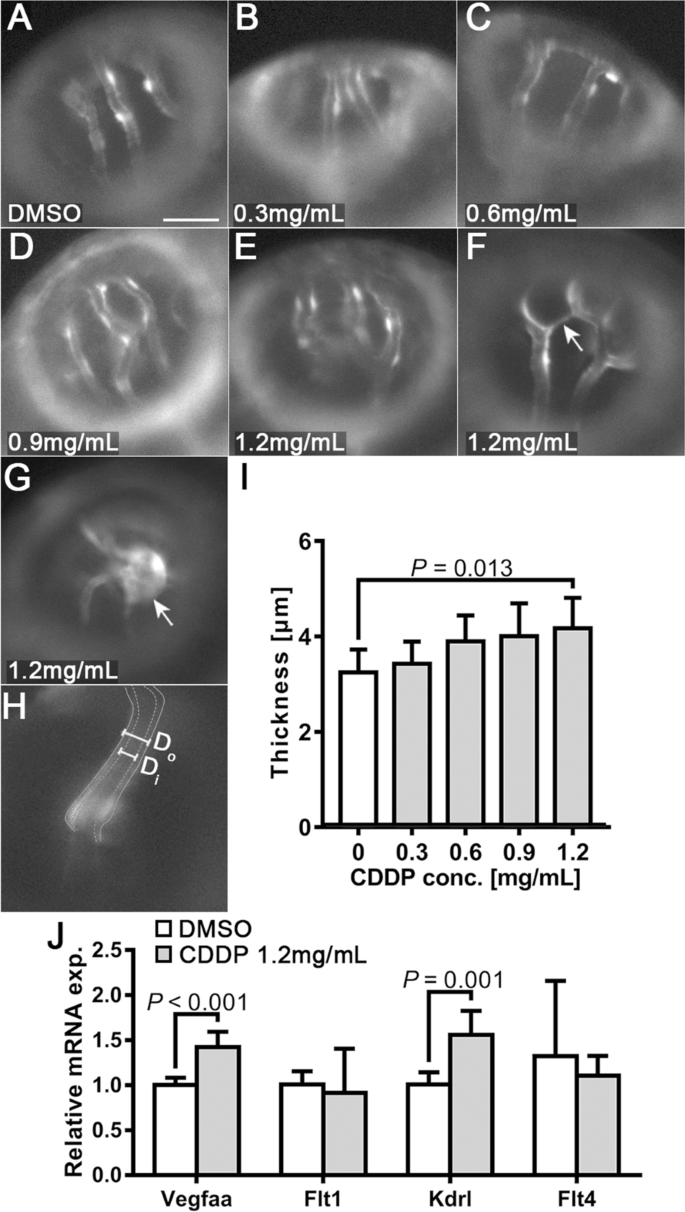
Endothelial layer thickening and ectopic growth of optical vessels in the CDDP treatment group. A-E Different treatments with CDDP led to thickening of the endothelial layer in the optical arteries of Tg(fli1:EGFP) larvae. Scale bar, 50 μm. In the 1.2 mg/mL CDDP treatment group, ectopic sprouts F and tumor-like growth G could be observed. H Endothelial layer thickness (T) was calculated by subtracting inner diameter (Di) from outer diameter (Do) using the formula T = (Do—Di)/2. I Changes in the thickness of the endothelial layer were quantified and are presented as the mean ± SEM. CDDP promoted thickening of the endothelial layer, although only the 1.2 mg/mL group showed statistical significance. N = 15 for each group, and the experiment was replicated 4 times. J Twenty-four hpf Tg(fli1: EGFP) embryos treated with or without CDDP (1.2 mg/mL) for 48 h. The mRNA extracted from 20 embryos from the same group was pooled, and the expressions of VEGF receptors (Flt1, Kdrl and Flt4) and VEGF (Vegfaa) were analyzed by RT–qPCR. Both VEGF receptor 2 (Vegfaa) and Kdrl were significantly upregulated in the CDDP treatment group. The relative expression level of each gene was normalized to the fold change compared with the control group. N = 6, and the data are presented as the mean ± SEM
Full size image
Analysis of target genes involved in FGF, MAPK/ERK, and SREBP 1 and 2 signaling pathways in CDDP-treated embryos
Other pathways involved in angiogenesis were also detected. Previous studies have revealed the important role of the FGF signaling pathway in regulating angiogenesis in zebrafish [22]. However, examination of the expression of the key genes showed that the 1.2 mg/mL CDDP treatment exhibited no obvious regulation of the FGF pathway (Fig. 4A). However, both bRaf and MAP kinase interacting serine/threonine kinase 2 (Mknk2) play a role in regulating the MAPK/ERK signaling pathway, which might next regulate angiogenesis. Figure 4B shows no discernible difference in the relative mRNA expression levels of the genes involved in this pathway in CDDP-exposed zebrafish embryos, except JUN, a crucial target gene of the MAPK/ERK signaling pathway. The upregulation of JUN was consistent with the previous prediction in Fig. 1. Due to the reported effect of Radix Salviae on lipid metabolism, two key signaling pathways, SREBP 1 and 2, were further detected. The results showed that the SREBP 2, but not the SREBP 1, pathway was significantly activated. As a target gene of the SREBP 2 pathway, Fasn was also upregulated (Fig. 4C).
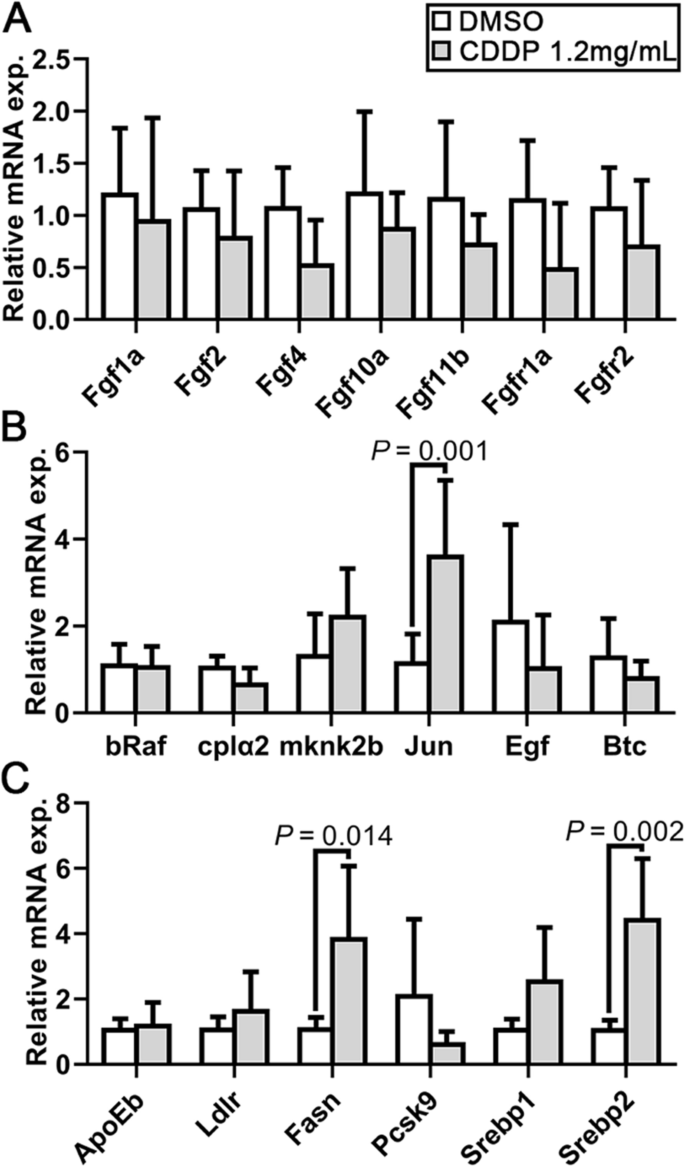
Analysis of FGF, MAPK/ERK, SREBP 1 and 2 signaling pathways in CDDP-treated embryos. Expression levels were quantified using RT–qPCR and normalized against β-actin. Data are represented by relative fold changes ± SEM with respect to control embryos (arbitrarily set to 1). A Relative mRNA expression levels of Fgf1a, Fgf2, Fgf4, Fgf10a and Fgf11b, as well as the receptors Fgfr1a and Fgfr2. None were statistically changed. B The relative mRNA expression of bRaf, cplα2, mknk2b, JUN, Egf and Btc was also detected. Only JUN expression was significantly upregulated. C Relative mRNA expression levels of the SREBP 1 and 2 signaling pathways showed that Srebp2 might be activated and that as a downstream factor, Fasn expression was elevated. N = 6, and the data are presented as the mean ± SEM
Full size image
Proangiogenesis effect of CDDP in VRI-induced ISV deficiency in zebrafish
Lenvatinib is a specific VEGF receptor inhibitor (VRI) and can be used to establish a defective angiogenesis model in zebrafish [20]. In the present study, 24 hpf embryos were pretreated with VRI (100 nM) for 24 h and then treated with 1.2 mg/mL CDDP or fresh VRI for another 24 h (Fig. 5A). We found that CDDP treatment caused obvious enlargement of the posterior blood island (PBI), and that ISV growth was inhibited by VRI with decreased intact vessels and increased defective vessels (Fig. 5B-C). However, CDDP recovered the VRI-impaired ISV growth in zebrafish (Fig. 5B-C). The ISV index was used to quantify the growth of ISVs, which was calculated using the formula ISV index = (intact vessel number × 1) + (defective vessel number × 0.5). Consistent with the results above, VRI treatment significantly reduced the ISV index, whereas CDDP restored it (Fig. 5D).
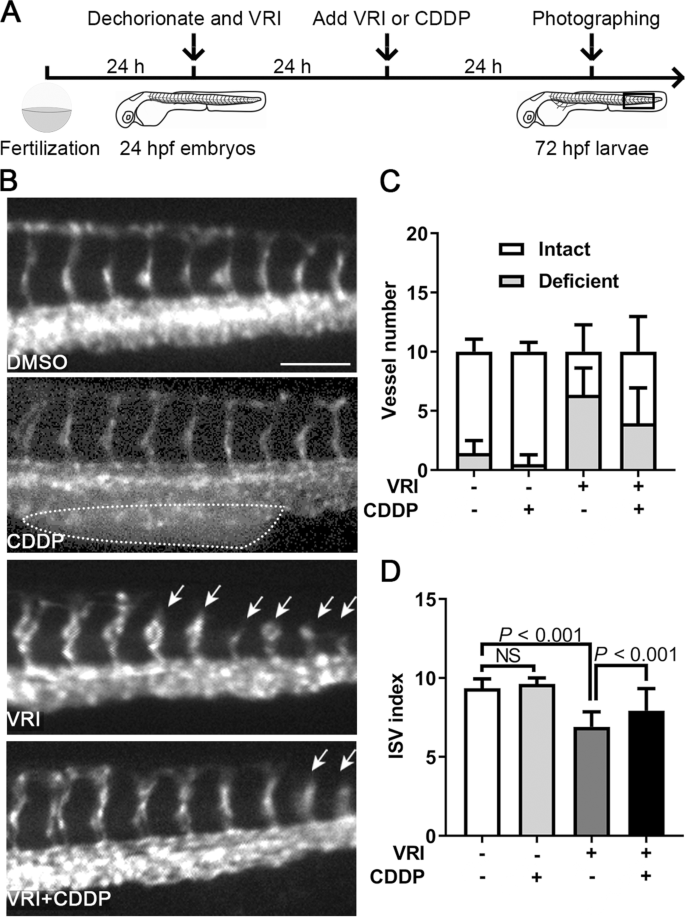
Pro-angiogenesis effect of CDDP in VRI-induced ISV deficiency in zebrafish. A Schematic representation of the experimental design. Twenty-four hpf Tg(fli1:EGFP) zebrafish embryos were pretreated with VRI (100 nM) for 24 h and then treated with 1.2 mg/mL CDDP or fresh VRI for another 24 h. Ten consecutive ISVs in the framed area were photographed, observed in each embryo, and then displayed in B. Fifteen embryos were included in each group, and the experiment was replicated three times. B Embryos treated with CDDP alone showed obvious enlarged posterior blood islands (PBIs, indicated by the dotted circle), whereas VRI treatment inhibited the development of ISVs, which could be restored by CDDP treatment. Arrows indicate deficient ISVs. Scale bar, 100 μm. C The numbers of intact and deficient vessels were calculated. D The ISV index was used to quantify the growth of ISVs, which was calculated using the formula ISV index = (intact vessel number × 1) + (defective vessel number × 0.5). The results showed that there was no obvious defect in the development of ISVs in the CDDP and DMSO treatment groups, but the ISVs of the VRI-treated embryos were significantly shorter, and the VRI + CDDP treatment reversed the phenotype. N = 15, and the data are presented as the mean ± SEM
Full size image
PI3K and AKT inhibitors reduced the proangiogenic effect of CDDP in zebrafish.
It has been reported that the VEGF receptor activates the PI3K/AKT signaling pathway and promotes angiogenesis [20]. Our screening results above also suggested that the VEGF/PI3K/AKT signaling pathway probably affected angiogenesis, so we speculated that CDDP also promotes the angiogenesis process by activating the VEGF/PI3K/AKT signaling pathway. Based on this inference, because CDDP reversed the vascular development disorder caused by VRI, when the activation of the PI3K/AKT pathway was inhibited, the protective effect of CDDP on vascular development might also be blocked. Hence, the PI3K inhibitor LY (0.3–3 µM) and AKT inhibitor AKTi (0.03–0.3 µM) were employed to evaluate this prediction in vivo (Fig. 6A). Consistent with previous reports, we found that PI3K and AKT inhibitors reduced the proangiogenic effect of CDDP (1.2 mg/mL) in a VRI-induced angiogenesis deficiency model in a dose-dependent manner (Fig. 6B). Interestingly, the growth of ISVs in embryos treated with PI3K and AKT inhibitors but without CDDP or VRI was slightly stimulated (Fig. 6C). Moreover, PI3K and AKT inhibitors hindered the proangiogenic effect of CDDP even without VRI pretreatment (Fig. 6D).
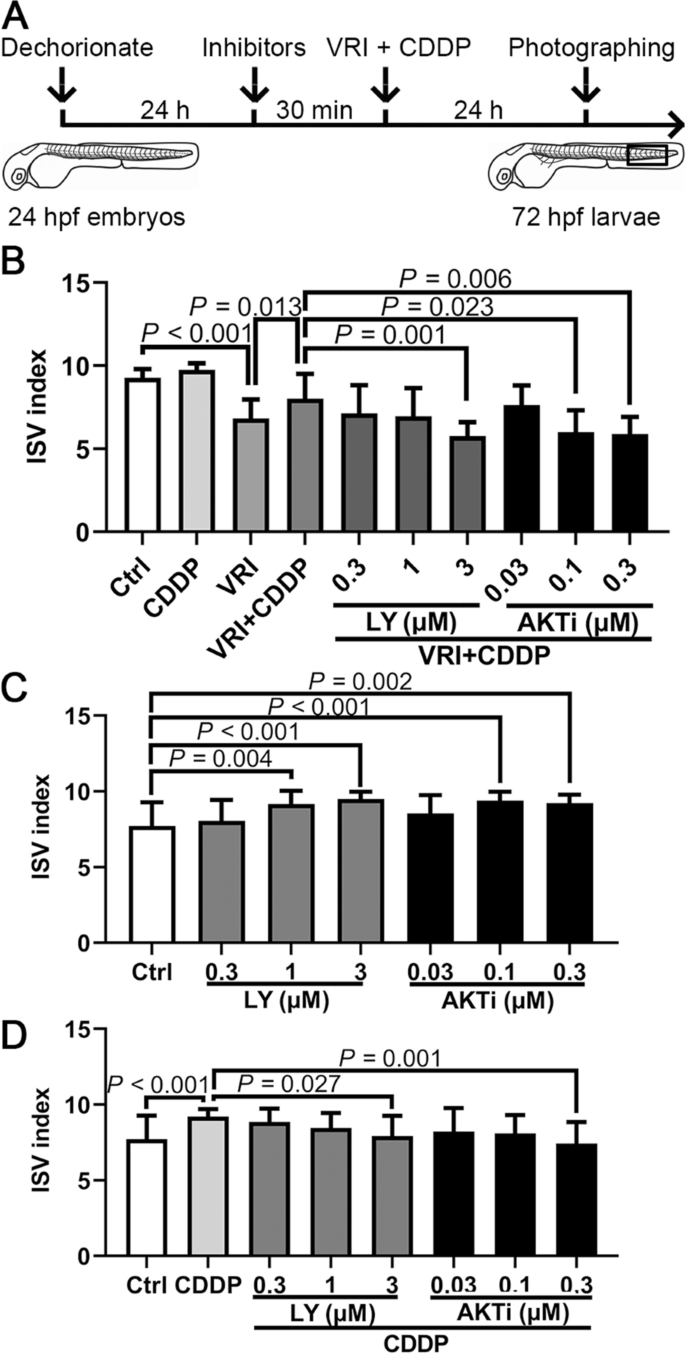
The effect of the VEGF/PI3K/AKT signaling pathway on the proangiogenic effect of CDDP. A Schematic representation of the experimental design. Twenty-four hpf Tg(fli1:EGFP) embryos were pretreated with various concentrations of the PI3K inhibitor LY294002 (LY, 0.3–3 µM) and the AKT inhibitor VIII (AKTi, 0.03–0.3 µM) for 30 min and were then cotreated with VRI (100 nM) and CDDP (1.2 mg/mL) for 24 h. Ten consecutive ISVs in the framed area were observed in each embryo. Fifteen embryos were analyzed in each group, and the experiment was replicated four times. B CDDP relieved the abnormal development of ISVs, which could be inhibited by 3 µM LY, 0.1 or 0.3 µM AKTi. C Twenty-four hpf embryos were treated with various concentrations of LY (0.3–3 µM) and AKTi (0.03–0.3 µM) for 24 h. Both inhibitors could promote angiogenesis of ISVs at specific concentrations. D The angiogenic efficacy of CDDP could be weakened by LY or AKTi at specific concentrations. The ISV index of each zebrafish embryo was calculated as (the number of intact vessels × 1) + (the number of defective vessels × 0.5). Data are presented as the mean ± SEM
Full size image
Discussion
As a novel field that integrates bioinformatics with pharmacology, biochemistry, and genomics [23], network pharmacology is an extraordinary tool to visualize and analyze complex interactive data on herbs, compounds, targets, and diseases based on computer modeling analysis and target prediction methods [24] and is particularly suitable for characterizing TCM formulations. For example, CDDP is a TCM formula with complex components. There may be a variety of synergistic effects among its active ingredients, which play a role in the treatment of CHD. Therefore, it is impractical to extract and study the effective compounds it contains one by one. In this study, we took advantage of network pharmacology and predicted the compounds contained in the two main components of CDDP, Radix Salviae and Panax notoginseng, and their regulatory targets. We compared these target genes with the reported genes related to CHD and screened 65 potential targets of CDDP in the treatment of CHD (Fig. 1A). Further studies showed that CDDP might indeed promote angiogenesis because some key genes and signaling pathways in the process of angiogenesis were predicted to be targets of CDDP, such as the VEGFA and PI3K/AKT pathways (Fig. 1). VEGFA binds to the VEGF receptor (VEGFR), particularly VEGFR2 (Kdrl), and then mediates downstream pathways to regulate angiogenesis. The PI3K/AKT signaling pathway was also suggested to regulate endothelial cell proliferation, migration, and invasion, therefore promoting angiogenesis [20].
We then employed the transgenic zebrafish line Tg(fli1:EGFP), which expresses enhanced fluorescent protein in vascular endothelial cells, to test whether CDDP actually has proangiogenic activity in vivo and in the involved pathways. This animal model, with the advantages of high genetic similarity to humans and low cost, was convenient for vascular system observation under a fluorescence microscope. We found that CDDP did not affect the survival rate or early global development below a concentration of 1.2 mg/mL (Fig. 2A-E), which indicates low lethal toxicity. When focused on the vascular system, we noticed that CDDP significantly promoted SIV branching and sprouting (Fig. 2G-I). Simultaneously, OA demonstrated a thickened endothelial layer (Fig. 3A-E) and ectopic development (Fig. 3F-G) after CDDP treatment. The RT–qPCR assays revealed that the VEGF/VEGFR signaling pathway was affected (Fig. 3J). These results indicate that CDDP promotes SIV and OA growth in zebrafish.
Several reported key genes and pathways affecting angiogenesis such as the FGF and MAPK/ERK pathways were detected (Fig. 4A-B). However, their expression was largely unchanged after CDDP treatment, except JUN, which was also predicted to be activated by CDDP, as shown in Fig. 1. This might be another target gene of CDDP in CHD treatment and is worthy of further study. Notably, the lipid-metabolism-related SREBP2 pathway was upregulated (Fig. 4C). This indicated that CDDP might have a lipid-lowering effect, and this might be another potential mechanism in CHD treatment. Further confirmation was achieved through inhibitor-induced ISV injury experiments. The enlarged PBI that we observed in CDDP-treated embryos suggested that CDDP may also promote the differentiation and proliferation of the hematopoietic endothelium. Therefore, CDDP might also promote early hematopoiesis in zebrafish embryos, which suggests that it could also be a potential drug for the treatment of blood system diseases, which is worthy of further exploration. Our results showed that VRI significantly impaired ISV sprouting, whereas CDDP could rescue the phenotype (Fig. 5). We have proven through in vivo experiments and VEGF/VEGFR pathway gene expression analysis that CDDP activates this pathway to promote angiogenesis. As a well-known and widely studied pathway involved in angiogenesis across a variety of vertebrates, VEGF/VEGFR signaling was reported to regulate various downstream targets. Fan et al. reported that Dalbergia odorifera extract has a proangiogenic effect, and its mechanism could be VEGF/VEGFR mRNA regulation and PI3K/MAPK signaling pathway activation [20]. Because the PI3K/AKT signaling pathway was also predicted by our network pharmacological study to be a potential key target pathway of CDDP in the treatment of CHD, we further tested whether the PI3K/AKT signaling pathway played a role downstream of the CDDP promotion of the angiogenesis process. Consistent with our speculation, when cotreated with LY or AKTi, the restoration of ISV growth induced by CDDP was clearly inhibited (Fig. 6B), which proved that PI3K/AKT might be the potential pathway of CDDP’s proangiogenic effect. In brief, we hypothesize that CDDP activates PI3K, a downstream molecule of the VEGF/VEGFR pathway, by increasing the expression of VEGFA and Kdrl, thereby increasing the phosphorylation of AKT1 and exerting transcriptional regulation to promote vascular endothelial proliferation and angiogenesis (Supplemental Fig. S1). Interestingly, when treated with LY or AKTi alone, ISV growth was slightly enhanced (Fig. 6C). This might be because angiogenesis is regulated by multiple signaling pathways. When PI3K/AKT alone was inhibited, some other pathways may be regulated in vivo to some extent, maintaining a physiological balance of angiogenesis. Direct treatment with CDDP also promoted ISV development, which could be diminished by LY or AKTi cotreatment (Fig. 6D). These results further proved that CDDP might be used to treat CHD through VEGF/VEGFR and PI3K/AKT pathway-mediated angiogenesis.
However, our study still has many limitations. For example, fold changes of the genes and pathways mentioned in this manuscript was not further detected on the protein level due to the lack of specific antibodies against zebrafish. Hence, our results could provide a reference for TCM researchers and clinicians but still need further verification
Conclusions
In conclusion, our study demonstrates that CDDP, a patented TCM formula, promotes angiogenic effects in zebrafish embryos through the activation of the VEGF/VEGFR and PI3K/AKT signaling pathways. Our results provide new evidence for the application of CDDP in the treatment of CHD.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AKT:
-
Protein kinase B
- AKT1:
-
V-AKT murine thymoma viral oncogene homologue 1
- AKTi:
-
AKT inhibitor VIII
- ANOVA:
-
Analysis of variance
- AP:
-
Angina pectoris
- bRaf:
-
B-Raf proto-oncogene, serine/threonine kinase
- CDDP:
-
Compound Danshen Dripping Pill
- CFDA:
-
China Food and Drug Administration
- CHD:
-
Coronary heart disease
- CMD:
-
Cardiac microvascular disorder
- CVD:
-
Cardiovascular disease
- Di:
-
Inner diameter
- DL:
-
Drug-likeness
- DMSO:
-
Dimethyl sulphoxide
- Do:
-
Outer diameter
- EC:
-
Endothelial cell
- EGFP:
-
Enhanced green fluorescent protein
- ERK:
-
Extracellular regulated protein kinase
- Fasn:
-
Fatty acid synthase
- FDA:
-
US Food and Drug Administration
- FGF:
-
Fibroblast growth factor
- Flt1:
-
Fms related receptor tyrosine kinase 1
- Flt4:
-
Fms-related tyrosine kinase 4
- GO:
-
Gene ontology
- hpf:
-
Hours post fertilization
- ISV:
-
Intersegmental vessel
- UN:
-
Jun proto-oncogene
- AP-1:
-
Transcription factor subunit
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- Kdrl:
-
Vascular endothelial growth factor receptor kdr-like
- LC50:
-
The median lethal concentration;
- LY:
-
LY294002
- MAPK:
-
Mitogen-activated protein kinase
- Mknk2:
-
MAP kinase interacting serine/threonine kinase 2
- OA:
-
Optic artery
- OB:
-
Oral bioavailability
- PI3K:
-
Phosphatidylinositol 3-kinase
- PPI:
-
Protein–protein interaction
- PTU:
-
2-Phenylthiourea;
- rt-qPCR:
-
Real-time quantitative PCR
- SEM:
-
Standard error of mean
- SIV:
-
Sub-intestinal vessel plexus
- SREBP:
-
Sterol regulatory element binding protein
- T:
-
Thickness of endothelial layer
- TCM:
-
Traditional Chinese medicine
- TCMSP:
-
Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform
- VEGF:
-
Vascular endothelial growth factor
- VEGFA:
-
Vascular endothelial growth factor A
- VEGFR:
-
Vascular endothelial growth factor receptor
- VRI:
-
VEGF receptor kinase inhibitor Lenvatinib
- WT:
-
Wild-type; w/v, weight/volume
References
-
Zhu KF, Wang YM, Zhu JZ, Zhou QY, Wang NF. National prevalence of coronary heart disease and its relationship with human development index: A systematic review. Eur J Prev Cardiol. 2016;23(5):530–43.
Article
Google Scholar
-
Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis. 2017;20(2):185–204.
Article
Google Scholar
-
Zhou ZY, Huan LY, Zhao WR, Tang N, Jin Y, Tang JY. Spatholobi Caulis extracts promote angiogenesis in HUVECs in vitro and in zebrafish embryos in vivo via up-regulation of VEGFRs. J Ethnopharmacol. 2017;200:74–83.
Article
CASGoogle Scholar
-
Sajib S, Zahra FT, Lionakis MS, German NA, Mikelis CM. Mechanisms of angiogenesis in microbe-regulated inflammatory and neoplastic conditions. Angiogenesis. 2018;21(1):1–14.
Article
CASGoogle Scholar
-
Potz BA, Parulkar AB, Abid RM, Sodha NR, Sellke FW. Novel molecular targets for coronary angiogenesis and ischemic heart disease. Coron Artery Dis. 2017;28(7):605–13.
Article
Google Scholar
-
Tasly Pharmaceuticals: Phase III Trial of Dantonic® (T89) Capsule to Prevent and Treat Stable Angina (CAESA) https://www.clinicaltrials.gov/ct2/show/NCT01659580 (2017). Assessed 5 Jan 2021.
-
Lv C, Liu C, Liu J, Li Z, Du X, Li Y, et al. The Effect of Compound Danshen Dripping Pills on the Dose and Concentration of Warfarin in Patients with Various Genetic Polymorphisms. Clin Ther. 2019;41(6):1097–109.
Article
CASGoogle Scholar
-
Yang XY, He K, Pan CS, Li Q, Liu YY, Yan L, et al. 3, 4-dihydroxyl-phenyl lactic acid restores NADH dehydrogenase 1 α subunit 10 to ameliorate cardiac reperfusion injury. Sci Rep. 2015;5:10739.
Article
CASGoogle Scholar
-
Zhang Y, Zhao J, Ding R, Niu W, He Z, Liang C. Pre-treatment with compound Danshen dripping pills prevents lipid infusion-induced microvascular dysfunction in mice. Pharm Biol. 2020;58(1):701–6.
Article
CASGoogle Scholar
-
Chen K, Wang C, Fan Y, Gu J, Han Z, Wang Y, et al. Identification of mundoserone by zebrafish in vivo screening as a natural product with anti-angiogenic activity. Exp Ther Med. 2018;16(6):4562–8.
CAS
PubMed
PubMed CentralGoogle Scholar
-
Chen K, Fan Y, Gu J, Han Z, Zeng H, Mao C, et al. In vivo Screening of Natural Products Against Angiogenesis and Mechanisms of Anti-Angiogenic Activity of Deoxysappanone B 7,4’-Dimethyl Ether. Drug Des Devel Ther. 2020;14:3069–78.
-
Wu Y, Xu S, Tian XY. The Effect of Salvianolic Acid on Vascular Protection and Possible Mechanisms. Oxid Med Cell Longev. 2020;2020:5472096.
PubMed
PubMed CentralGoogle Scholar
-
Zhou Z-Y, Xiao Y, Zhao W-R, Zhang J, Shi W-T, Ma Z-L, et al. Pro-angiogenesis effect and transcriptome profile of Shuxinyin formula in zebrafish. Phytomedicine. 2019;65:153083.
Article
Google Scholar
-
Wang Z, Mao Y, Cui T, Tang D, Wang XL. Impact of a Combined High Cholesterol Diet and High Glucose Environment on Vasculature. PLoS ONE. 2013;8(12):e81485.
Article
Google Scholar
-
Wang W, Ru S, Wang L, Qin J, Ru Y, Zhang J, et al. Bisphenol S Induces Ectopic Angiogenesis in Embryos via VEGFR2 Signaling, Leading to Lipid Deposition in Blood Vessels of Larval Zebrafish. Environ Sci Technol. 2020;54(11):6822–31.
Article
CASGoogle Scholar
-
Kanehisa M, Furumichi M, Sato Y, Ishiguro-Watanabe M, Tanabe M. KEGG: integrating viruses and cellular organisms. Nucleic Acids Res. 2021;49(D1):D545–51.
Article
CASGoogle Scholar
-
Sun G, Song Y, Li L, Hou Z, Tong L. Quickly quantifying the dissolution fingerprints of compound Danshen dropping pill by HPLC. Ann Transl Med. 2013;1(2):16.
PubMed
PubMed CentralGoogle Scholar
-
Chen J, Zhu RF, Li FF, Liang YL, Wang C, Qin YW, et al. MicroRNA-126a Directs Lymphangiogenesis Through Interacting With Chemokine and Flt4 Signaling in Zebrafish. Arterioscler Thromb Vasc Biol. 2016;36(12):2381–93.
Article
CASGoogle Scholar
-
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310.
Article
CASGoogle Scholar
-
Fan ZM, Wang DY, Yang JM, Lin ZX, Lin YX, Yang AL, et al. Dalbergia odorifera extract promotes angiogenesis through upregulation of VEGFRs and PI3K/MAPK signaling pathways. J Ethnopharmacol. 2017;204:132–41.
Article
CASGoogle Scholar
-
Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130(21):5281–90.
Article
CASGoogle Scholar
-
Nicoli S, De Sena G, Presta M. Fibroblast growth factor 2-induced angiogenesis in zebrafish: the zebrafish yolk membrane (ZFYM) angiogenesis assay. J Cell Mol Med. 2009;13(8B):2061–8.
Article
Google Scholar
-
Liu B, Song Z, Yu J, Li P, Tang Y, Ge J. The atherosclerosis-ameliorating effects and molecular mechanisms of BuYangHuanWu decoction. Biomed Pharmacother. 2020;123:109664.
Article
CASGoogle Scholar
-
Li H, Liu L, Liu C, Zhuang J, Zhou C, Yang J, et al. Deciphering Key Pharmacological Pathways of Qingdai Acting on Chronic Myeloid Leukemia Using a Network Pharmacology-Based Strategy. Med Sci Monit. 2018;24:5668–88.
Article
CASGoogle Scholar
Download references
Acknowledgements
We thank Prof. Qing Jing’s lab members for technical support
Funding
The work described in this paper is supported by Youth Startup Fund of Shanghai Changhai Hospital (2019QNB10).
Author information
Author notes
-
Yang-Xi Hu, Hong-Min You and Chang-Zhen Ren contributed equally to this work.
Authors and Affiliations
-
Department of Cardiology, Changzheng Hospital, Naval Medical University, Shanghai, 200003, China
Yang-Xi Hu, Chang-Zhen Ren, Bo-Wen Hu, Yan-Da Zhang, Zhi-Qing He, Ru Ding & Chun Liang
-
Department of Cardiology, Changhai Hospital, Naval Medical University, Shanghai, 200433, China
Hong-Min You, Lu-Jun Zhang & Zhi-Fu Guo
Authors
- Yang-Xi Hu
You can also search for this author inPubMed Google Scholar
- Hong-Min You
You can also search for this author inPubMed Google Scholar
- Chang-Zhen Ren
You can also search for this author inPubMed Google Scholar
- Bo-Wen Hu
You can also search for this author inPubMed Google Scholar
- Lu-Jun Zhang
You can also search for this author inPubMed Google Scholar
- Yan-Da Zhang
You can also search for this author inPubMed Google Scholar
- Zhi-Qing He
You can also search for this author inPubMed Google Scholar
- Ru Ding
You can also search for this author inPubMed Google Scholar
- Zhi-Fu Guo
You can also search for this author inPubMed Google Scholar
- Chun Liang
You can also search for this author inPubMed Google Scholar
Contributions
YXH and HMY designed and carried out the experiments. YXH and CZR analyzed experimental results and perform the procedures of bioinformatics. LJZ organized figures and tables. YXH wrote the manuscript. YDZ and BWH provided technical assistance. ZQH and RD provided languish assistance. ZFG supervised the experiments. CL administrated the project and reviewed the writing & editing.
Corresponding authors
Correspondence to
Zhi-Fu Guo or Chun Liang.
Ethics declarations
Ethics approval and consent to participate
The experimental protocol was established, according to the ethical guidelines and was approved by the Ethics Committee of Animal Experiments (ECAE), Shanghai Changhai Hospital (approval no. 2020026). All methods were performed in accordance with the relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
All authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Fig. S1. CDDP promotes sprouting angiogenesis. Schematic diagram of CDDP promoting angiogenesis by activating the VEGF/VEGFR and PI3K/AKT signaling pathways.
Additional file 2. Table 1.
Composition of CDDP. The main 2 components ofCDDP are listed in the table.
Additional file 3. Table 2.
Primers usedfor RT–qPCR in this study. The primers used in this study are listed in thetable.
Additional file 4.
Table 3. Pharmacokinetic parameters of theingredients reported in Radix Salviae. The 65 ingredients and their pharmacokinetic parameters in RadixSalviae were obtained from the online database TCMSP.
Additional file 5. Table 4.
Pharmacokinetic parameters of theingredients reported for Panax notoginseng. The 8 ingredients and their pharmacokinetic parameters in Panaxnotoginseng were obtained from the online database TCMSP.
Additional file 6. Table 5.
Genes related to active ingredients of Radix Salviae. The screening of ingredient-related genes of Radix Salviae wasperformed using TCMSP and confirmed through the DrugBank database
Additional file 7.
Table 6. Genes related to the active ingredients of Panax notoginseng. The screening of ingredient-related genes of Panax notoginsengwas performed using TCMSP and confirmed through the DrugBank database.
Additional file 8.
Table 7. Key genes related to coronary heart disease.The 7,100 coronary heart disease-related genes reported were screened outthrough the GeneCards database
Additional file 9.
Table 8. Screenedtarget genes of CDDP for CHD treatment. In total, 65 potential target genes of CDDP forCHD treatment were identified.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article
Hu, YX., You, HM., Ren, CZ. et al. Proangiogenesis effects of compound danshen dripping pills in zebrafish.
BMC Complement Med Ther 22, 112 (2022). https://doi.org/10.1186/s12906-022-03589-y
Download citation
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1186/s12906-022-03589-y
Keywords
1. Introduction
Stable angina pectoris (SAP) is one of the most common manifestations of coronary artery disease (CAD) (1). A survey reported that the prevalence of chronic SAP was 7.7% in Iran (2). A prospective study showed that 20% of patients with CAD had symptoms related with angina pectoris, which may be associated with an increased risk of adverse cardiovascular outcomes (3). A cohort study found that the high frequency of angina pectoris was associated with the high incidence of secondary cardiovascular events and death in patients with stable coronary heart disease (4).
At present, the goals of treatments for SAP mainly focus on the reduction in cardiovascular adverse events and the improvement in the quality of life (5). There are many antianginal therapies, such as nitrates, beta-blockers, calcium channel blockers and ranolazine (5). Nitrates as standard antianginal treatments have been used in the management of angina pectoris for many years (6). A systematic review found that nitrates could significantly reduce the angina attacks and improve the exercise duration (6). However, some adverse events associated with nitrates are reported, such as headache and dizziness (6). Moreover, long-term use may lead to patients’ tolerance to nitrates and the reduction of protection duration (7).
Traditional Chinese medicine (TCM) is beneficial for the management of angina pectoris. A systematic review showed that TCM could reduce the frequency of angina pectoris, shorten the duration of angina attack and improve the quality of life (8). Compound danshen dropping pills (CDDP) is a TCM formula, and is composed of Salvia miltiorrhiza Bunge, Panax notoginseng (Burkill) F.H. Chen and Dryobalanops aromatica C.F. Gaertn. TCM network pharmacology is a new approach to understanding herb formula (9, 10). A study based on the network pharmacology and molecular docking found that the mechanisms of CDDP for treating SAP might be associated with the regulation of inflammatory response, apoptosis signal pathway, etc. (11). Some clinical trials comparing CDDP with nitrates for SAP have been published in recent years. However, the relative advantages and disadvantages of CDDP compared with nitrates for SAP were not systematically evaluated. Therefore, we conducted this systematic review to critically assess the efficacy and safety of CDDP vs. nitrates for SAP.
2. Methods
This study was registered on PROSPERO (No. CRD42022352888) available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022352888. It followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (12).
2.1. Inclusion and exclusion criteria
2.1.1. Type of included studies
Randomized controlled trials (RCTs) were considered for inclusion, regardless of publication date and language. Quasi-RCTs, cross-over trials, on-going trials, abstracts and letters were excluded.
2.1.2. Patients
Patients diagnosed with SAP were included. Age, gender, race and nationality were unrestricted. SAP should be assessed according to the internationally recognized criteria or guidelines, such as National Institute for Health and Care Excellence or European Society of Cardiology guidelines (13).
2.1.3. Interventions
CDDP was used to treat SAP in the experimental group. Nitrates were used for the management of SAP in the control group. According to the instruction of CDDP, patients should take CDDP ten pills a time, and three times a day for at least 4 weeks. The dosage, frequency and course of nitrates were unlimited. The types of nitrates such as isosorbide dinitrate and isosorbide nitrate were also unrestricted. Other specific interventions for treating SAP were inhibited in the two groups. Patients with SAP may be accompanied with other diseases, such as coronary heart disease. Conventional therapies for the management of these accompanied diseases were unlimited.
2.1.4. Outcomes
The primary outcomes were the effective rate and markedly effective rate in symptom improvement (14). The secondary outcomes included the effective rate and markedly effective rate in electrocardiogram (ECG) improvement, and adverse drug reactions. The patients were labeled as “effective” in symptom improvement when the frequency of angina attacks and nitroglycerin consumption were reduced by more than 50%, and “markedly effective” in symptom improvement when symptoms associated with SAP were disappeared, the frequency of angina attacks or nitroglycerin consumption was reduced by more than 80% or 90% after treatment. The patients were labeled as “effective” in ECG improvement when S-T segment was elevated by 0.5 mm, however, not recovered completely after treatment, or defined by guidelines. The patients were labeled as “markedly effective” in ECG improvement when resting electrocardiogram returned to normal level after treatment, or defined by guidelines. The effective rate is equal to the number of patients labeled as “effective” or “markedly effective” divided by the total number of patients in one group. The markedly effective rate is equal to the number of patients labeled as “markedly effective” divided by the total number of patients in one group.
2.2. Search strategy
Two reviewers (JZ and MZ) independently searched PubMed, Embase, Web of Science, Cochrane library, China National Knowledge Infrastructure, Wanfang Digital Periodicals, and Chinese Science and Technology Periodicals database from inception to April 2023. The search terms included (“compound danshen dripping pills” OR “fufang danshen diwan” OR “Cardiotonic Pills” OR “dantonic” OR T89) AND (nitrate OR “isosorbide dinitrate” OR “isosorbide nitrate”) AND (“stable angina” OR “angina” OR “angina pectoris” OR “stenocardia” OR “angor pectoris”). Moreover, ClinicalTrials.gov, World Health Organization International Clinical trials Registry platform, and Chinese Clinical Trial Registry were also be searched to identify potentially grey literatures. The detailed search strategies are attainable in Supplementary Material.
2.3. Study selection
The studies collected from the comprehensive literature search were imported into EndNote X9 software to remove duplicates. Then, two reviewers (JZ and MZ) independently checked titles and abstracts to delete obviously ineligible studies according to the inclusion and exclusion criteria. They checked the full texts of the remaining studies to further identify eligible studies. Disagreements were handled in consultation with a third reviewer (YH). A PRISMA flow diagram was used to describe the screening process.
2.4. Data extraction
The important data were extracted and imported into Microsoft Excel 2013 by two authors (JZ and MZ) independently. The information included characteristic of included studies (first author, publication year, country, sample size, design), patients (age, gender, race, nationality), interventions (type, dosage, frequency, duration), and outcomes (primary and secondary outcomes). The information on risk of bias assessment (randomization, allocation, blinding, loss to follow-up) were also extracted synchronously.
2.5. Assessment of risk of bias
The risk of bias for included RCTs was assessed by the Cochrane “risk of bias” tool. The selection bias, performance bias, detection bias, attrition bias, and reporting bias for each included RCT was classified to low, high or unclear level. Two reviewers (JZ and MZ) were independently assessed the risk of bias. Any disagreement was resolved by a third author (YH).
2.6. Statistical analysis
Odds ratio (OR) with 95% confidence intervals (CIs) was used to estimate the effect size for the dichotomous variables. The meta-analysis with the random-effect model as the primary analysis was conducted by Review Manager 5.4.1 software. Then, the meta-analysis with the fixed-effect model was conducted as the sensitivity analysis. P < 0.05 indicated a statistically significant difference between the two groups. Subgroup analyses were conducted based on the duration. The publication bias was assessed by the funnel plot. The level of evidence for each outcome was assessed by the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system.
3. Results
3.1. Literature search
This systematic review identified 1,385 potentially eligible studies via the extensive search. Nine hundred and thirty-three duplicate studies were removed using EndNote software. Then, 396 irrelevant studies were deleted by checking the titles and abstracts. After checking full-texts, 29 studies were finally included for the statistical analysis. The process of screening eligible studies is described in Figure 1 (15–43).
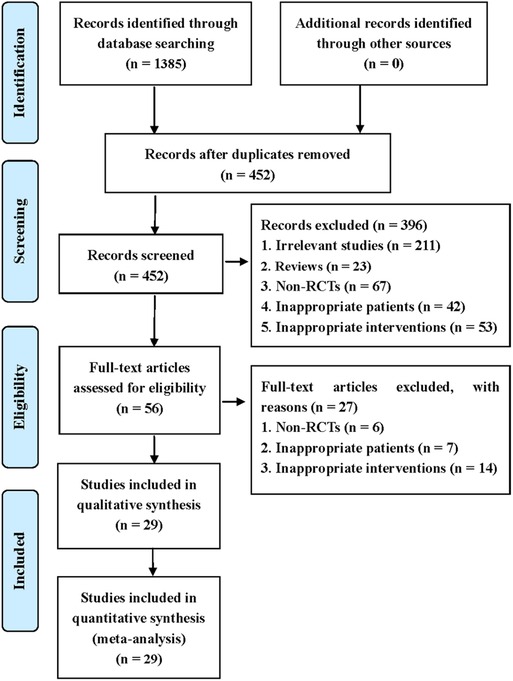
Figure 1. Flow diagram of study retrieval and selection.
3.2. Characteristics of included studies
The characteristics of the included studies are summarized in Table 1. They involved 3,185 patients and were published between 1997 and 2013. Sample size ranged from 25 to 120 in the experimental group and 25–114 in the control group. Isosorbide nitrate was used in 7 trials (15, 16, 19, 21, 23, 24, 42), and isosorbide dinitrate was used in other studies. There was the same dosage of CDDP or nitrates across the included studies. The duration of CDDP or nitrates ranged from 4 weeks to 12 weeks. However, patients took CDDP or nitrates with the same duration in the same study. Twenty-two RCTs reported the effective rate and the markedly effective rate in symptom improvement (17, 19–23, 26, 27, 29–42). Fifteen RCTs reported the effective rate and the markedly effective rate in ECG improvement (17, 22, 24, 26, 27, 29, 31–34, 36–39, 42). Twenty-three RCTs reported the adverse drug reactions (15–26, 28, 30, 32, 33, 35, 38–43).
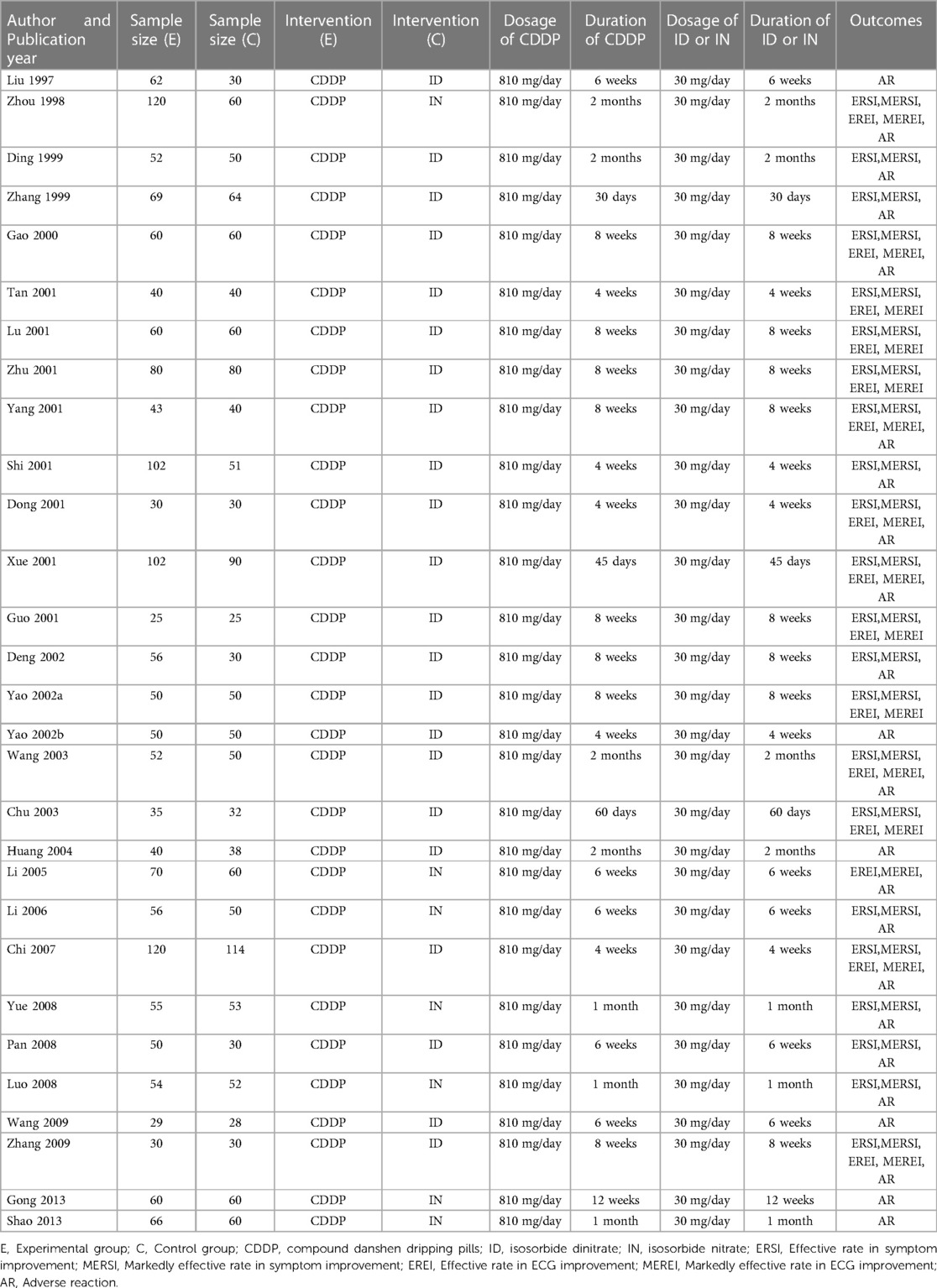
Table 1. Characteristics of included studies.
3.3. Assessment of risk of bias
Figures 2, 3 show the results of risk of bias assessment. The risk of selection bias was graded as unclear in all of the included studies because no specific random sequence generation and allocation concealment methods were reported. The risk of performance bias was also classified as unclear in all of the included studies because no blinding of participants, personnel or outcome assessment was clearly reported. The risk of attrition bias was low in all of the included studies due to the complete outcome data. The reporting bias and other bias were categorized as unclear risk because of the insufficient information.
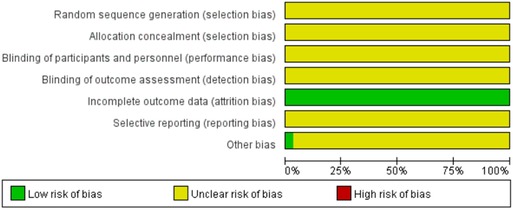
Figure 2. Risk of bias graph.
Figure 3. Risk of bias summary.
3.4. Symptom improvement
3.4.1. Effective rate in symptom improvement
Twenty-two RCTs reported the effective rate in symptom improvement. The meta-analyses with the random-effect model indicated that CDDP could significantly increase the effective rate in symptom improvement compared with nitrates (Pooled 9 RCTs, OR = 1.95, 95% CI: 1.25–3.05, P = 0.003, duration of about 4 weeks; Pooled 4 RCTs, OR = 3.45, 95% CI: 1.84–6.48, P = 0.0001, duration of about 6 weeks; Pooled 13 RCTs, OR = 4.02, 95% CI: 2.14–7.57, P < 0.0001, duration of about 8 weeks; Figure 4). The results of the meta-analyses with fixed-effect model were similar with above results in Table 2. In addition, one RCT (29) reported that the effective rate in symptom improvement in the CDDP group was higher than that in the nitrates group (OR = 1.5, 95% CI: 0.68–3.31, P = 0.32) after 5 weeks of CDDP treatment.
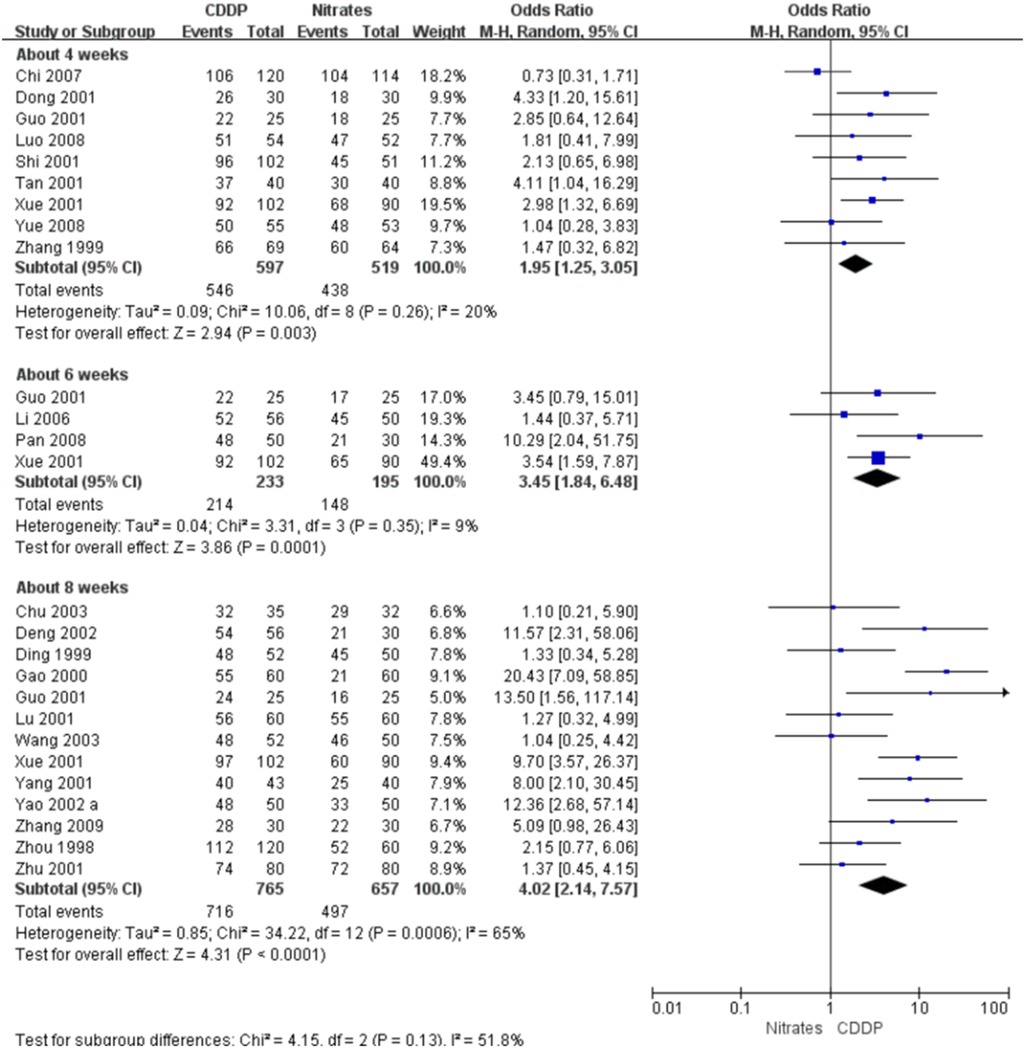
Figure 4. Meta-analyses on effective rate in symptom improvement.
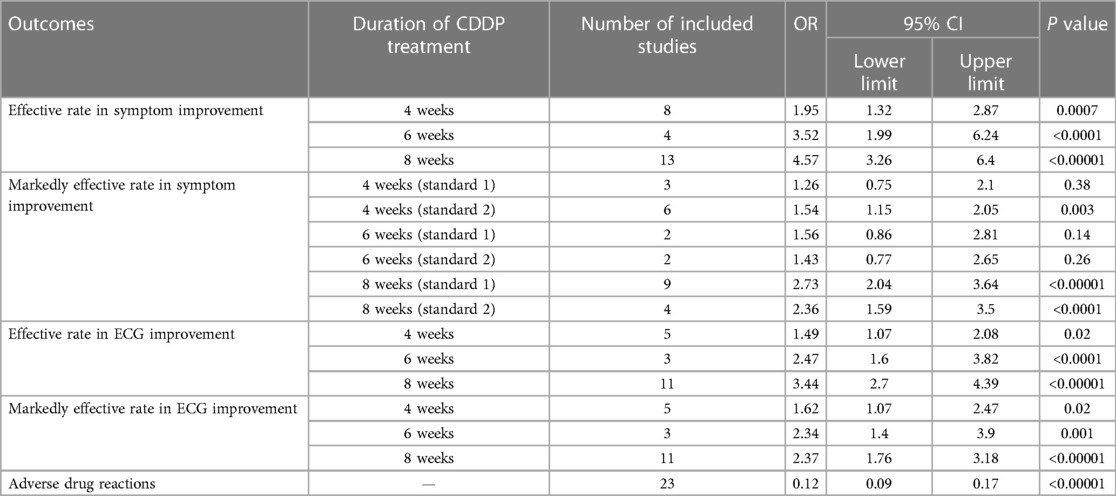
Table 2. Meta-analyses with fixed-effect model.
3.4.2. Markedly effective rate in symptom improvement
Twenty-two RCTs reported the markedly effective rate in symptom improvement. When the markedly effective in symptom improvement was defined as “symptoms associated with SAP were disappeared, the frequency of angina attacks or nitroglycerin consumption was reduced by more than 80% after treatment” (standard 1), the meta-analyses with the random-effect model indicated that CDDP compared with nitrates could increase the markedly effective rate in symptom improvement with no statistical significance at 4 and 6 weeks after treatment (Pooled 3 RCTs, OR = 1.26, 95% CI: 0.75–2.10, P = 0.38, duration of about 4 weeks; Pooled 2 RCTs, OR = 1.56, 95% CI: 0.86–2.81, P = 0.14, duration of about 6 weeks; Figure 5) and with the statistical significance at 8 weeks after treatment (Pooled 9 RCTs, OR = 2.63, 95% CI: 1.70–4.05, P < 0.0001, duration of about 8 weeks; Figure 5). When the markedly effective in symptom improvement was defined as “symptoms associated with SAP were disappeared, the frequency of angina attacks or nitroglycerin consumption was reduced by more than 90% after treatment” (standard 2), the meta-analyses with the random-effect model indicated that CDDP compared with nitrates could increase the markedly effective rate in symptom improvement with no statistical significance at 6 weeks after treatment (Pooled 2 RCTs, OR = 1.42, 95% CI: 0.76–2.65, P = 0.27, duration of about 6 weeks; Figure 6) and with the statistical significance at 4 and 8 weeks after treatment (Pooled 6 RCTs, OR = 1.55, 95% CI: 1.14–2.11, P = 0.005, duration of about 4 weeks; Pooled 4 RCTs, OR = 2.36, 95% CI: 1.59–3.50, P < 0.0001, duration of about 8 weeks; Figure 6). The results of the meta-analyses with fixed-effect model were similar with above results in Table 2.
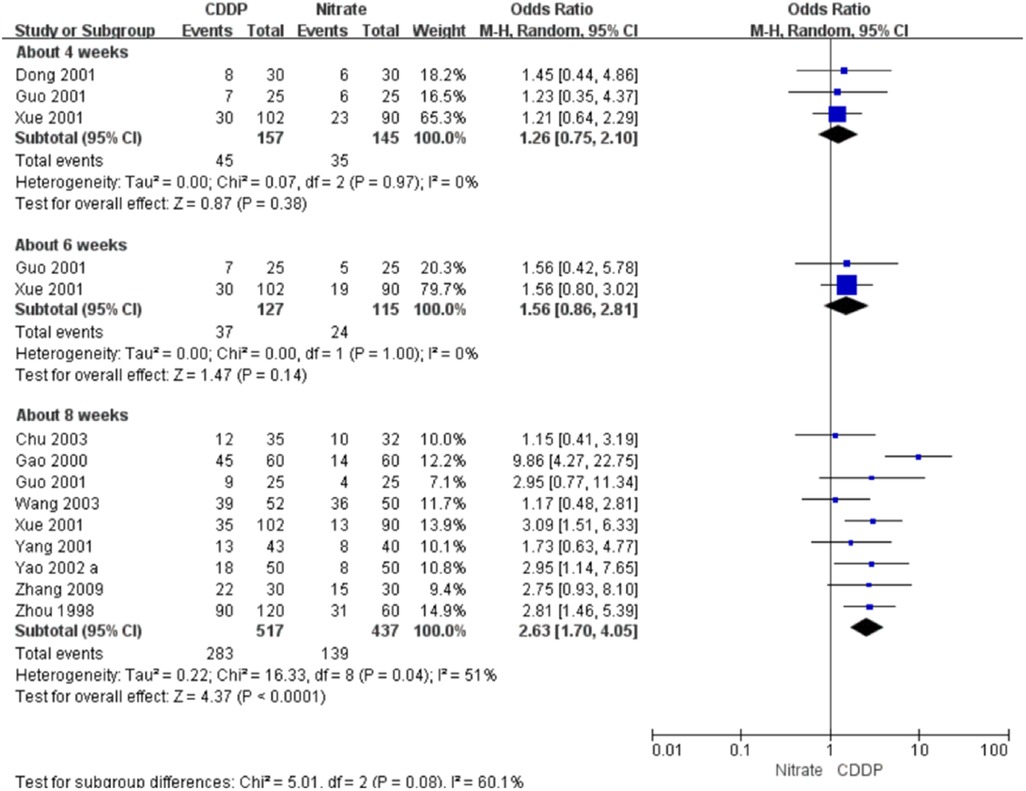
Figure 5. Meta-analyses on markedly effective rate in symptom improvement (standard 1).
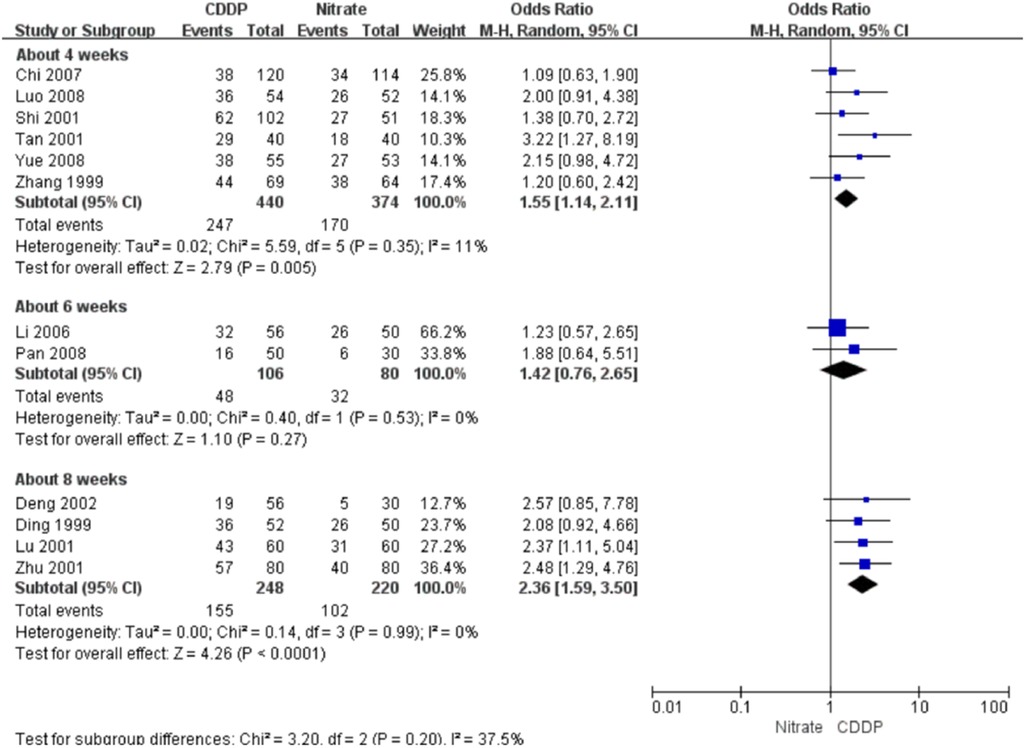
Figure 6. Meta-analyses on markedly effective rate in symptom improvement (standard 2).
3.5. ECG improvement
3.5.1. Effective rate in ECG improvement
Fifteen RCTs reported the effective rate in ECG improvement. The meta-analyses with the random-effect model indicated that CDDP could significantly increase the effective rate in ECG improvement compared with nitrates (Pooled 5 RCTs, OR = 1.60, 95% CI: 1.02–2.52, P = 0.04, duration of about 4 weeks; Pooled 3 RCTs, OR = 2.47, 95% CI: 1.60–3.82, P < 0.0001, duration of about 6 weeks; Pooled 11 RCTs, OR = 3.43, 95% CI: 2.68–4.38, P < 0.00001, duration of about 8 weeks; Figure 7). The results of the meta-analyses with fixed-effect model were similar with above results in Table 2.
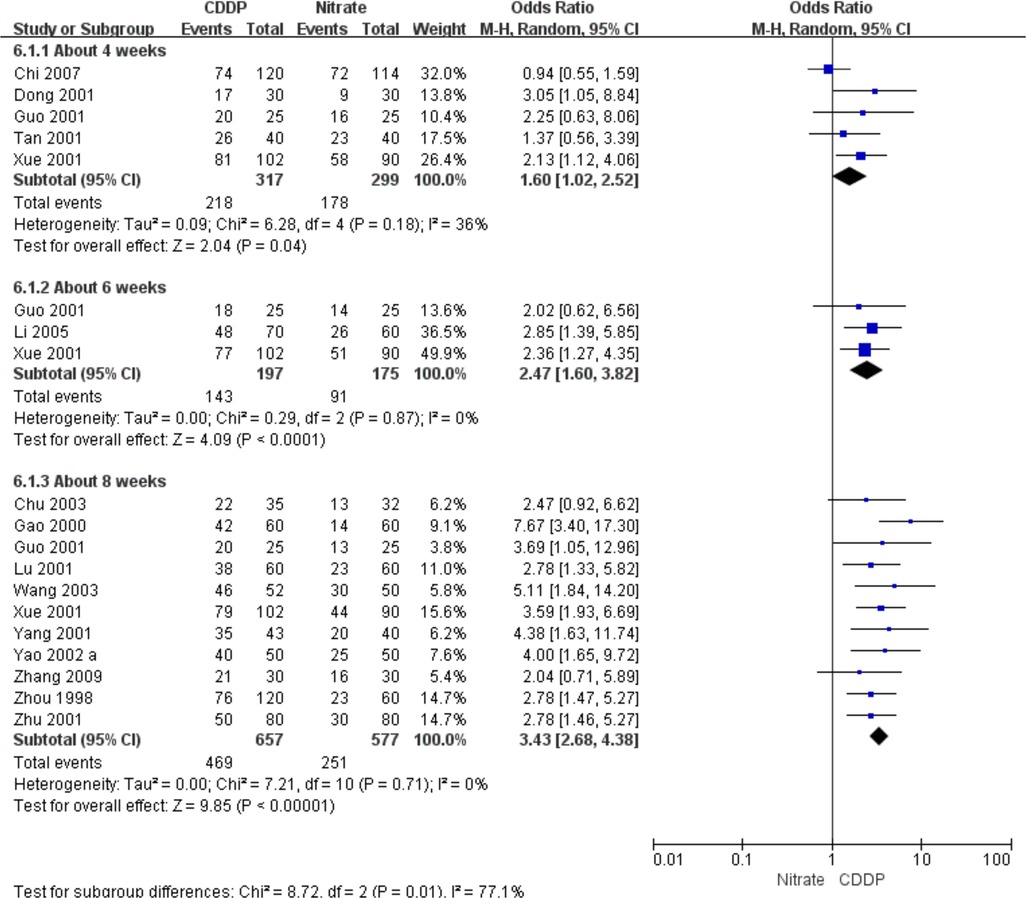
Figure 7. Meta-analyses on effective rate in ECG improvement.
3.5.2. Markedly effective rate in ECG improvement
Fifteen RCTs reported the markedly effective rate in ECG improvement. The meta-analyses with the random-effect model indicated that CDDP could significantly increase the markedly effective rate in ECG improvement compared with nitrates (Pooled 5 RCTs, OR = 1.62, 95% CI: 1.07–2.47, P = 0.02, duration of about 4 weeks; Pooled 3 RCTs, OR = 2.33, 95% CI: 1.39–3.90, P = 0.001, duration of about 6 weeks; Pooled 11 RCTs, OR = 2.35, 95% CI: 1.74–3.16, P < 0.00001, duration of about 8 weeks; Figure 8). The results of the meta-analyses with fixed-effect model were similar with above results in Table 2.
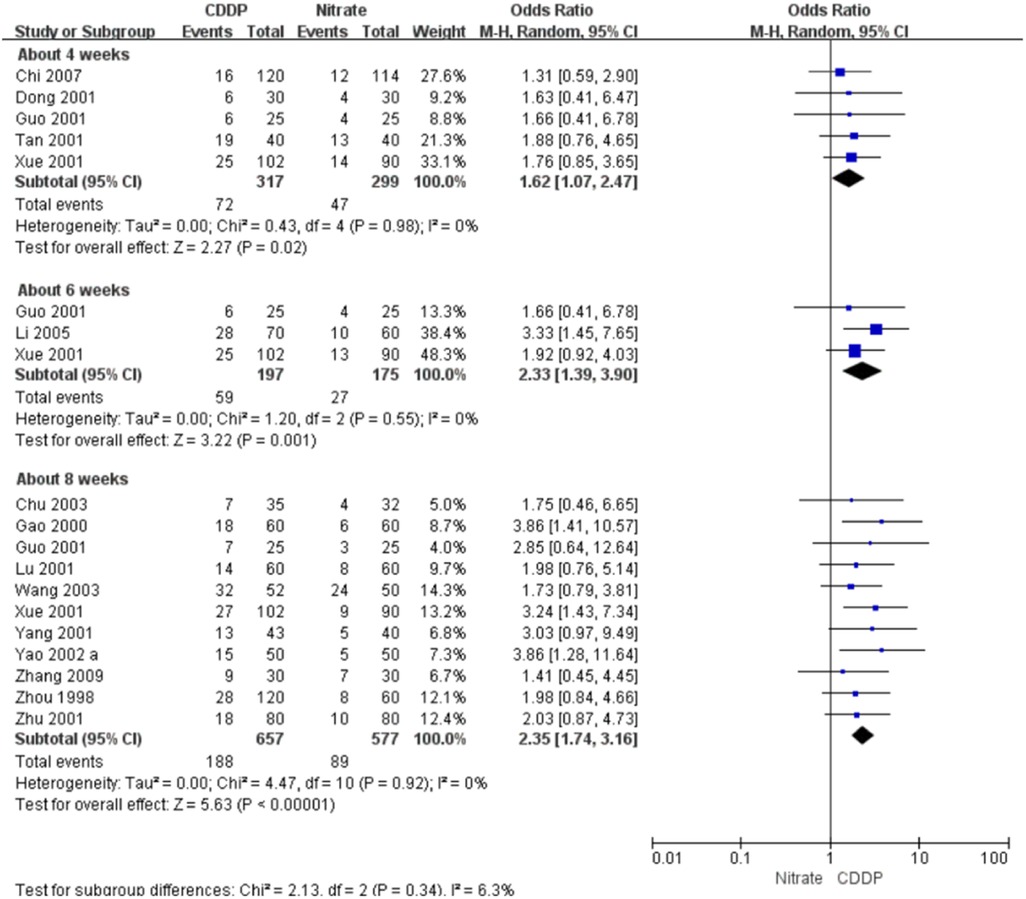
Figure 8. Meta-analyses on markedly effective rate in ECG improvement.
3.6. Adverse drug reactions
Twenty-three RCTs reported the adverse drug reactions. The pooled result showed that the incidence of adverse drug reactions in the CDDP group was lower than that in the nitrates group (Pooled 23 RCTs, OR = 0.15, 95% CI: 0.1–0.21, P < 0.00001, Figure 9). Specifically, adverse drug reactions included stomach discomfort in the CDDP group. In the nitrate group, adverse drug reactions included headache, dizziness, facial burning, nausea, etc.
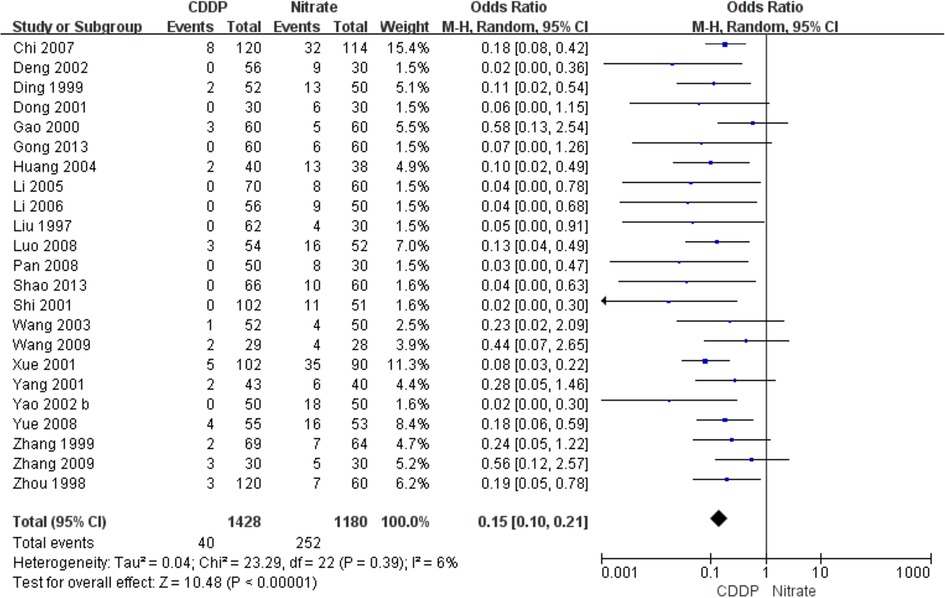
Figure 9. Meta-analysis on the adverse drug reactions.
3.7. Publication bias
Funnel plots of the meta-analyses are presented in Figure 10. Publication bias may be existed because of the asymmetry in some funnel plots. It may be associated with small sample size, poor methodological quality, etc.
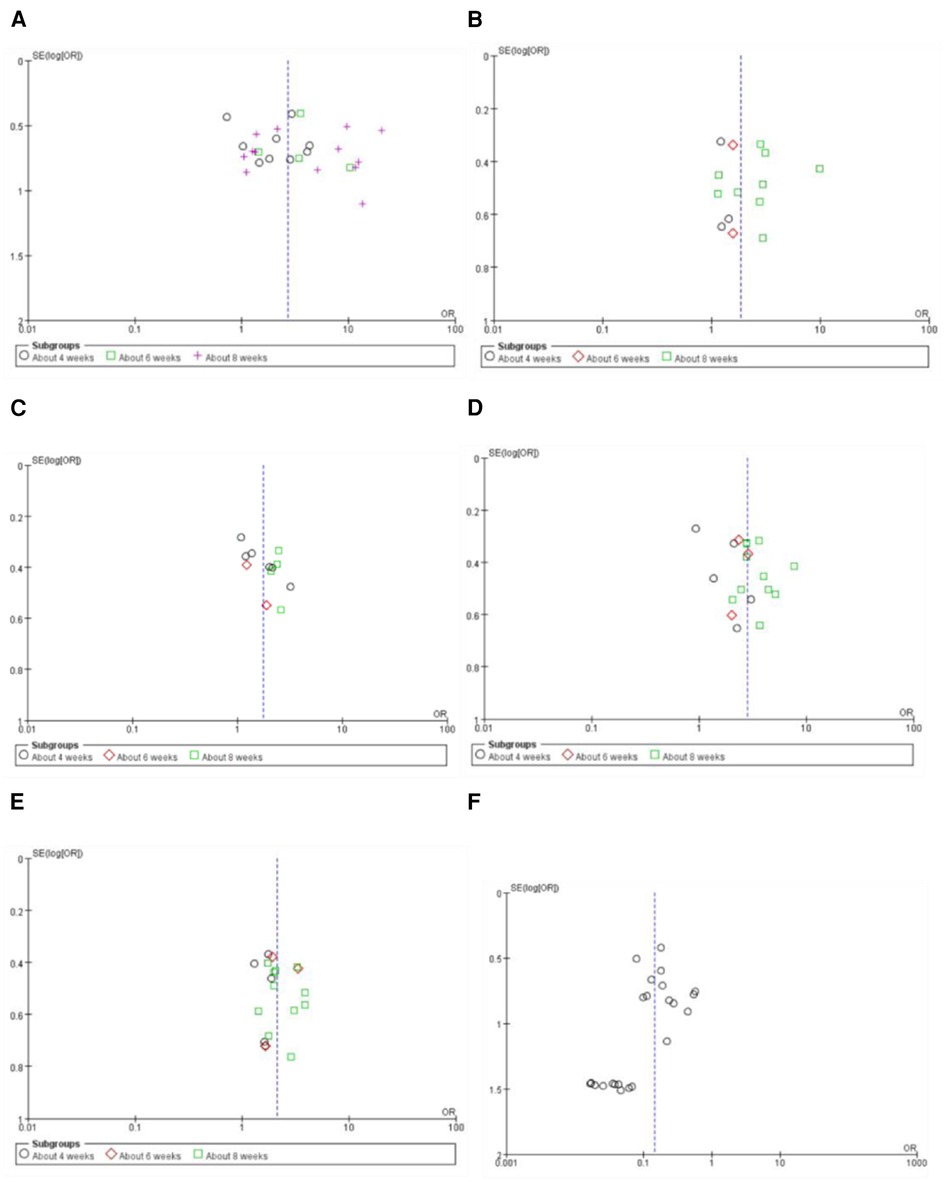
Figure 10. Funnel plot of the meta-analyses on effective rate in symptom improvement (A), markedly effective rate in symptom improvement (standard 1) (B), markedly effective rate in symptom improvement (standard 2) (C), effective rate in ECG improvement (D), markedly effective rate in ECG improvement (E), and the adverse drug reaction (F).
3.8. Grade of evidence
The grade of evidence for each outcome was presented in Table 3. The levels of the evidence for the effective rate and the markedly effective rate in symptom improvement ranged from very low to low. The levels of the evidence for the effective rate and markedly effective rate in ECG improvement were classified as low.
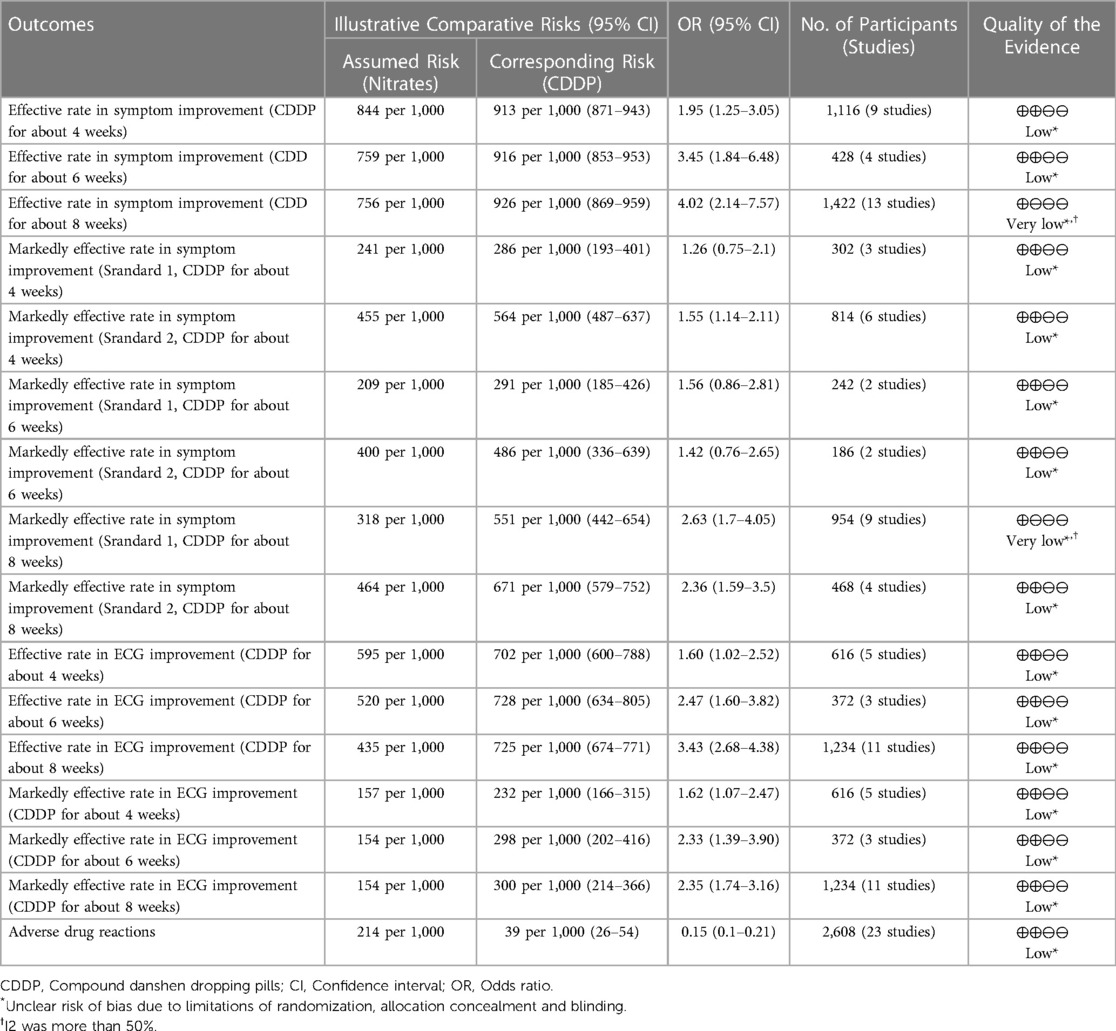
Table 3. GRADE quality of evidence summary table.
4. Discussion
Nitrates have been widely used in the management of angina in the clinical practice (44). However, the long-term use of nitrates may be limited because of the tolerance to nitrates (44). It may be caused by multiple factors, such as a burst of oxygen free radicals and vascular peroxynitrite formation (45–47). Many efforts are made to reduce the tolerance to nitrates, such as the intermittent nitrate therapy and long-acting nitrates (48). However, intermittent nitrate therapy may bring about the rebound ischemia (49), and long-acting nitrates may be associated with the endothelial dysfunction (48). At present, novel nitrate drugs are still under development (50, 51). Some side effects associated with nitrates for treating angina have been reported, such as headache, dizziness, and hypotension (52). Therefore, alternative therapies for angina are still needed.
CDDP has been approved for treating angina associated with coronary heart disease by the National Medical Products Administration in China for over twenty years. Many RCTs comparing CDDP with nitrates for treating angina has been published in recent years. A systematic review in 2015 suggested that CDDP were more effective than isosorbide dinitrate in improving the symptoms and ECG in patients with angina (53). However, some limitations on that study can’t be ignored. For example, only isosorbide dinitrate as one type of nitrates was considered as the control intervention. The safety was not assessed and the subgroup analysis based on stable and unstable angina was not conducted. Another systematic review in 2021 did not conduct subgroup analyses based on the duration of the drugs, and did not report adverse drug reactions (54). In this study, an updated systematic review with the strict eligibility criteria was conducted to critically evaluate the efficacy and safety of CDDP vs. nitrates for treating SAP. The meta-analyses were conducted based on the duration of CDDP. The pooled results showed that the effective rate in symptom improvement and ECG improvement were significantly increased at about 4, 6 or 8 weeks after CDDP treatment compared to nitrates. The incidence of adverse reactions in the CDDP group was lower than that in the nitrates group. It means that CDDP with the duration of at least 4 weeks has the relative advantages in relieving symptoms associated with SAP, reducing the frequency of angina attacks or nitroglycerin consumption, and has relatively fewer adverse effects compared with nitrates. It provides a new insight into the management of SAP. CDDP with the duration of at least 4 weeks may be considered as an alternative to nitrates for treating SAP.
Several limitations should be taken into consideration when interpreting above results. Firstly, the effect size may be overestimated or underestimated due to the small sample size (less than 100) in most of included studies. Secondly, the risks of selection bias, performance bias, reporting bias and other bias for most of included studies were graded as unclear because of insufficient information. Thirdly, the mechanisms of CDDP for treating SAP aren’t fully understood due to the lack of pharmacological evidence.
5. Conclusion
The present study suggests that CDDP with the duration of at least 4 weeks can be considered as an alternative to nitrates for treating SAP. However, more high-quality RCTs are still needed to confirm these findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
MZ: methodology, investigation, data curation, formal analysis, writing—original draft, writing—review and editing. WW: writing—original draft, writing—review and editing. HS: writing—original draft, writing—review and editing. JZ: conceptualization, methodology, investigation, data curation, writing—original draft, writing—review and editing. YH: conceptualization, methodology, investigation, data curation, writing—original draft, writing—review and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
MZ, WW, HS and YH were employed by Tasly Pharmaceutical Group Co., Ltd. HS was also employed by Tasly Holding Group Co., Ltd.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1168730/full#supplementary-material.
References
2. Abbasi M, Neishaboury M, Koohpayehzadeh J, Etemad K, Meysamie A, Asgari F, et al. National prevalence of self-reported coronary heart disease and chronic stable angina pectoris: factor analysis of the underlying cardiometabolic risk factors in the SuRFNCD-2011. Glob Heart. (2018) 13(2):73–82 e1. doi: 10.1016/j.gheart.2018.01.001
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Steg PG, Greenlaw N, Tendera M, Tardif JC, Ferrari R, Al-Zaibag M, et al. Prospective observational longitudinal registry of patients with stable coronary artery disease I. Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the international observational CLARIFY registry. JAMA Intern Med. (2014) 174(10):1651–9. doi: 10.1001/jamainternmed.2014.3773
PubMed Abstract | CrossRef Full Text | Google Scholar
4. Beatty AL, Spertus JA, Whooley MA. Frequency of angina pectoris and secondary events in patients with stable coronary heart disease (from the heart and soul study). Am J Cardiol. (2014) 114(7):997–1002. doi: 10.1016/j.amjcard.2014.07.009
PubMed Abstract | CrossRef Full Text | Google Scholar
6. Wei J, Wu T, Yang Q, Chen M, Ni J, Huang D. Nitrates for stable angina: a systematic review and meta-analysis of randomized clinical trials. Int J Cardiol. (2011) 146(1):4–12. doi: 10.1016/j.ijcard.2010.05.019
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Thadani U. Challenges with nitrate therapy and nitrate tolerance: prevalence, prevention, and clinical relevance. Am J Cardiovasc Drugs. (2014) 14(4):287–301. doi: 10.1007/s40256-014-0072-5
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Chen W, Wang B, Ge Y, Xu H, Jiang C, Yu P, et al. A systematic review and meta-analysis of clinical research on treating angina pectoris of coronary heart disease with traditional Chinese medicine to promote blood circulation and remove blood stasis. Ann Palliat Med. (2021) 10(10):10506–14. doi: 10.21037/apm-21-2233
PubMed Abstract | CrossRef Full Text | Google Scholar
10. Li S. Mapping ancient remedies: applying a network approach to traditional Chinese medicine. Science. (2015) 350(6262):S72–4.
Google Scholar
11. Chen K, Chen M, Wang Q, Dong J, Ma S, Zhao X, et al. Potential molecular mechanism of compound danshen dripping pills in treatment of angina pectoris based on network pharmacology and molecular docking. Yaowu Pingjia Yanjiu. (2022) 45(7):1282–93.
Google Scholar
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
CrossRef Full Text | Google Scholar
13. Archbold RA. Comparison between national institute for health and care excellence (NICE) and European society of cardiology (ESC) guidelines for the diagnosis and management of stable angina: implications for clinical practice. Open Heart. (2016) 3(1):e000406. doi: 10.1136/openhrt-2016-000406
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Li S, Wang R, Zhang Y, Zhang X, Layon AJ, Li Y, et al. Symptom combinations associated with outcome and therapeutic effects in a cohort of cases with SARS. Am J Chin Med (Gard City N Y). (2006) 34(6):937–47. doi: 10.1142/S0192415X06004417
CrossRef Full Text | Google Scholar
15. Shao Z, Ji L, Zhang H. Effect of compound danshen dripping pills on stable angina pectoris. People’s Military Surgeon. (2013) 56(4):423–4.
Google Scholar
16. Gong L. Curative effect of compound danshen dripping pills on 60 cases of stable angina pectoris. Chin J Geriatr Care. (2013) 11(2):54–5. doi: 10.3969/j.issn.1672-4860.2013.02.028
CrossRef Full Text | Google Scholar
17. Zhang S. Therapeutic effect of compound danshen dripping pills on stable angina pectoris in the elderly. J Changchun Univ Chin. (2009) 25(1):89. doi: 10.1007/s11802-009-0089-6
CrossRef Full Text | Google Scholar
18. Wang J. Curative effect of compound dasnhen dripping pills on 29 cases of stable angina pectoris. J Shanxi Univ Chin Med. (2009) 10(2):32–3. doi: 10.3969/j.issn.1671-0258.2009.02.013
CrossRef Full Text | Google Scholar
19. Yue X. Effect of compound danshen dripping pills on stable angina pectoris of coronary heart disease. J Mod Med Health. (2008) 24(19):2888–9.
Google Scholar
20. Pan C. Effect of compound Salvia miltiorrhiza dripping pills on stable angina pectoris. Tianjin Pharm. (2008) 20(4):56–7. doi: 10.3969/j.issn.1006-5687.2008.04.029
CrossRef Full Text | Google Scholar
21. Luo J. Clinical observation of compound danshen dripping pills in the treatment of stable angina pectoris. China Med Her. (2008) 5(22):82. doi: 10.3969/j.issn.1673-7210.2008.22
CrossRef Full Text | Google Scholar
22. Chi B, He T, Yang Y. Treatment of 120 cases of stable angina pectoris with compound danshen dripping pills. Shaanxi J Tradit Chin Med. (2007) 28(10):1283–5. doi: 10.3969/j.issn.1000-7369.2007.10.007
CrossRef Full Text | Google Scholar
23. Li Y, Xi Y, Zhou C, Fang L, Liu A. Effect of compound danshen dripping pills on stable angina pectoris. Clin J Med Off. (2006) 34(6):673–4. doi: 10.3969/j.issn.1671-3826.2006.06.004
CrossRef Full Text | Google Scholar
24. Li L, Yang H. Therapeutic effect of compound danshen dripping pills on 70 cases of stable angina pectoris. J Handan Med Coll. (2005) 18(1):34–5.
Google Scholar
25. Huang S, Zou X, Li P. Clinical observation of compound danshen dripping pills and nitrate in the treatment of stable angina pectoris. Mod Diagn Treat. (2004) 15(6):349–50. doi: 10.3969/j.issn.1001-8174.2004.06.013
CrossRef Full Text | Google Scholar
26. Wang X, Shen L, Yan L. Clinical analysis of compound danshen dripping pills in treatment of stable angina pectoris. J Inner Mongolia Med Univ. (2003) 25(3):204–5. doi: 10.3969/j.issn.1004-2113.2003.03.023
CrossRef Full Text | Google Scholar
27. Chu X, Geng J. Clinical analysis of compound danshen dripping pills in treatment of stable angina pectoris. Heilongjiang Med J. (2003) (4):16–7.
Google Scholar
28. Yao X. Clinical observation of compound danshen dripping pills in treating 50 cases of angina pectoris of coronary heart disease. J Emerg Tradit Chin. (2002) 11(1):26–7. doi: 10.3969/j.issn.1004-745X.2002
CrossRef Full Text | Google Scholar
29. Yao F, Zhang Y, Huang M, Liu H, Sun Y. Treatment of 50 cases of stable angina pectoris with compound danshen dripping pills. Shandong J Tradit Chin. (2002) 21(3):147–9. doi: 10.3969/j.issn.0257-358X.2002.03.011
CrossRef Full Text | Google Scholar
30. Deng G, He J, Li B. Treatment of stable angina pectoris with compound danshen dripping pills. Tianjin Pharm. (2002) 14(4):48. doi: 10.3969/j.issn.1006-5687.2002.04.029
CrossRef Full Text | Google Scholar
31. Zhu Y. Comparison of curative effect of compound danshen dripping pill combined with nitrate in treating stable angina pectoris. West J Tradit Chin Med. (2001) 2:52–3. doi: 10.3969/j.issn.1004-6852.2001.02.046
CrossRef Full Text | Google Scholar
32. Yang S, Huang T, Wang H. Effect of compound danshen dripping pills and nitrate on angina pectoris. J Wannan Med Coll. (2001) 20(3):198. doi: 10.3969/j.issn.1002-0217.2001.03.023
CrossRef Full Text | Google Scholar
33. Xue F, Liu S. Clinical observation of compound danshen dripping pills in the treatment of stable angina pectoris. J Hunan Univ Chin Med. (2001) 21(2):36–8. doi: 10.3969/j.issn.1674-070X.2001.02.016
CrossRef Full Text | Google Scholar
34. Tan S, Liu C. Clinical observation of compound danshen dripping pills in the treatment of stable angina pectoris. Heilongjiang Med J. (2001) 14(2):125. doi: 10.3969/j.issn.1006-2882.2001.02
CrossRef Full Text | Google Scholar
35. Shi Y, Zhang Z, Li R, Guan Y. Analysis of curative effect of compound danshen dripping pills on stable angina pectoris. Inner Mongolia J Tradit Chin. (2001) 05:6. doi: 10.16040/j.cnki.cn15-1101.2001.05.008
CrossRef Full Text | Google Scholar
36. Lu J, Sheng H. Treatment of stable angina pectoris with compound danshen dripping pills. West J Tradit Chin Med. (2001) 14(6):22–3. doi: 10.3969/j.issn.1004-6852.2001.06.016
CrossRef Full Text | Google Scholar
37. Guo L, Li S, Zhang W. Clinical observation of curative effect of compound danshen dripping pills on angina pectoris. J Shandong Med Coll. (2001) 23(2):113–5. doi: 10.3969/j.issn.1674-0947.2001.02.011
CrossRef Full Text | Google Scholar
38. Dong X, Zhang S. Effect of compound danshen dripping pills on stable angina pectoris. J Clin Med Pract. (2001) 2(3):143–5.
Google Scholar
39. Gao B, Wu Y. Curative effect of compound danshen dripping pills on 60 cases of stable angina pectoris. J Binzhou Med Univ. (2000) 23(1):68.
Google Scholar
40. Zhang G, Cao Y LUX, Liu Z, Liu W, Li W. Treatment of 69 cases of stable angina pectoris of coronary heart disease with compound danshen dripping pills. Jilin J Chin Med. (1999) 03:12.
Google Scholar
41. Ding X, Jia L, Wang C. Comparison of the efficacy of compound danshen dripping pills in the treatment of stable angina pectoris. Suzhou Univ J Med Sci. (1999) 19(5):512–3.
Google Scholar
42. Zhou Y. Treatment of 120 cases of stable angina pectoris with compound danshen dripping pills. Chin J New Drugs Clin Rem. (1998) 17(6):56–7.
Google Scholar
43. Liu Z, Gao S, Deng J, Li H. Effect of compound danshen dropping pills on angina pectoris of coronary heart disease. Chin Tradit Pat Med. (1997) 19(07):20–1+53.
Google Scholar
44. Klemenska E, Beręsewicz A. Bioactivation of organic nitrates and the mechanism of nitrate tolerance. Cardiol J. (2009) 16(1):11–9.19130411
PubMed Abstract | Google Scholar
45. Hink U, Oelze M, Kolb P, Bachschmid M, Zou M-H, Daiber A, et al. Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. J Am Coll Cardiol. (2003) 42(10):1826–34. doi: 10.1016/j.jacc.2003.07.009
PubMed Abstract | CrossRef Full Text | Google Scholar
47. Facchini E, Degiovanni A, Cavallino C, Lupi A, Rognoni A, Bongo AS. Beta-Blockers and nitrates pharmacotherapy and indications. Cardiovasc Hematol Agents Med Chem. (2015) 13(1):25–30. doi: 10.2174/1871525713666141219114708
PubMed Abstract | CrossRef Full Text | Google Scholar
49. Parker J. Potential problems with intermittent nitrate therapy. Can J Cardiol. (1996) 12(Suppl C):22C–4C. PMID: 8634920.8634920
PubMed Abstract | Google Scholar
50. Munzel T, Daiber A. Inorganic nitrite and nitrate in cardiovascular therapy: a better alternative to organic nitrates as nitric oxide donors? Vasc Pharmacol. (2018) 102:1–10. doi: 10.1016/j.vph.2017.11.003
CrossRef Full Text | Google Scholar
51. Liang J, Zhang P, Yang H, Zhang Y, Yao T, Liu K, et al. Design, synthesis and biological evaluation of novel nitric oxide donors with antioxidative activity. Eur J Med Chem. (2022) 236:114331. doi: 10.1016/j.ejmech.2022.114331
PubMed Abstract | CrossRef Full Text | Google Scholar
53. Yao Y, Feng Y, Lin W. Systematic review and meta-analysis of randomized controlled trials comparing compound danshen dripping pills and isosorbide dinitrate in treating angina pectoris. Int J Cardiol. (2015) 182:46–7. doi: 10.1016/j.ijcard.2014.12.112
PubMed Abstract | CrossRef Full Text | Google Scholar
54. Zheng Y, Wang X, Wang X. Pharmacoeconomic evaluation of compound danshen dripping pills and isosorbide dinitrate tablets in the treatment of stable angina pectoris based on meta—analysis. China Pharm. (2021) 30(12):87–91.
Google Scholar
